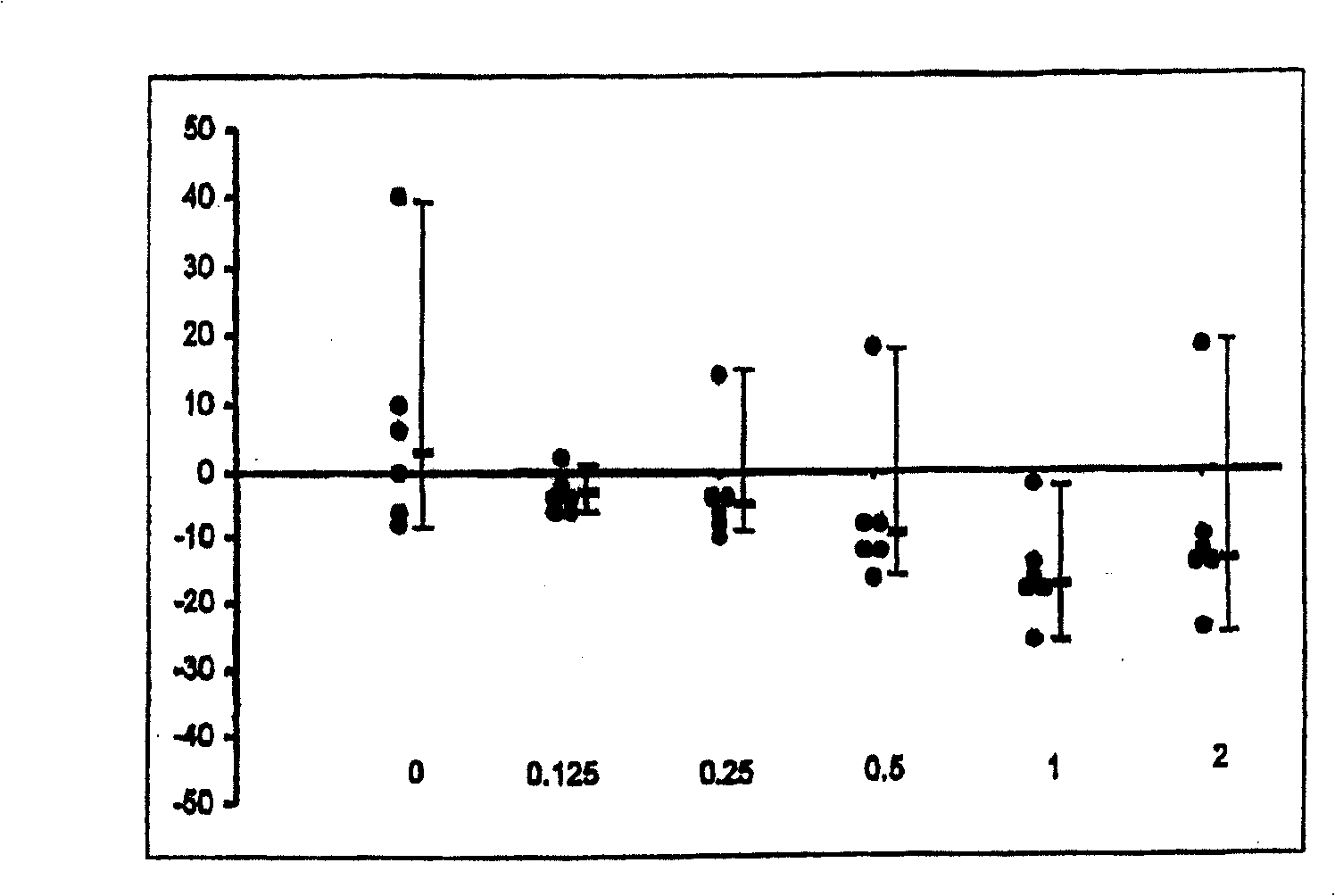
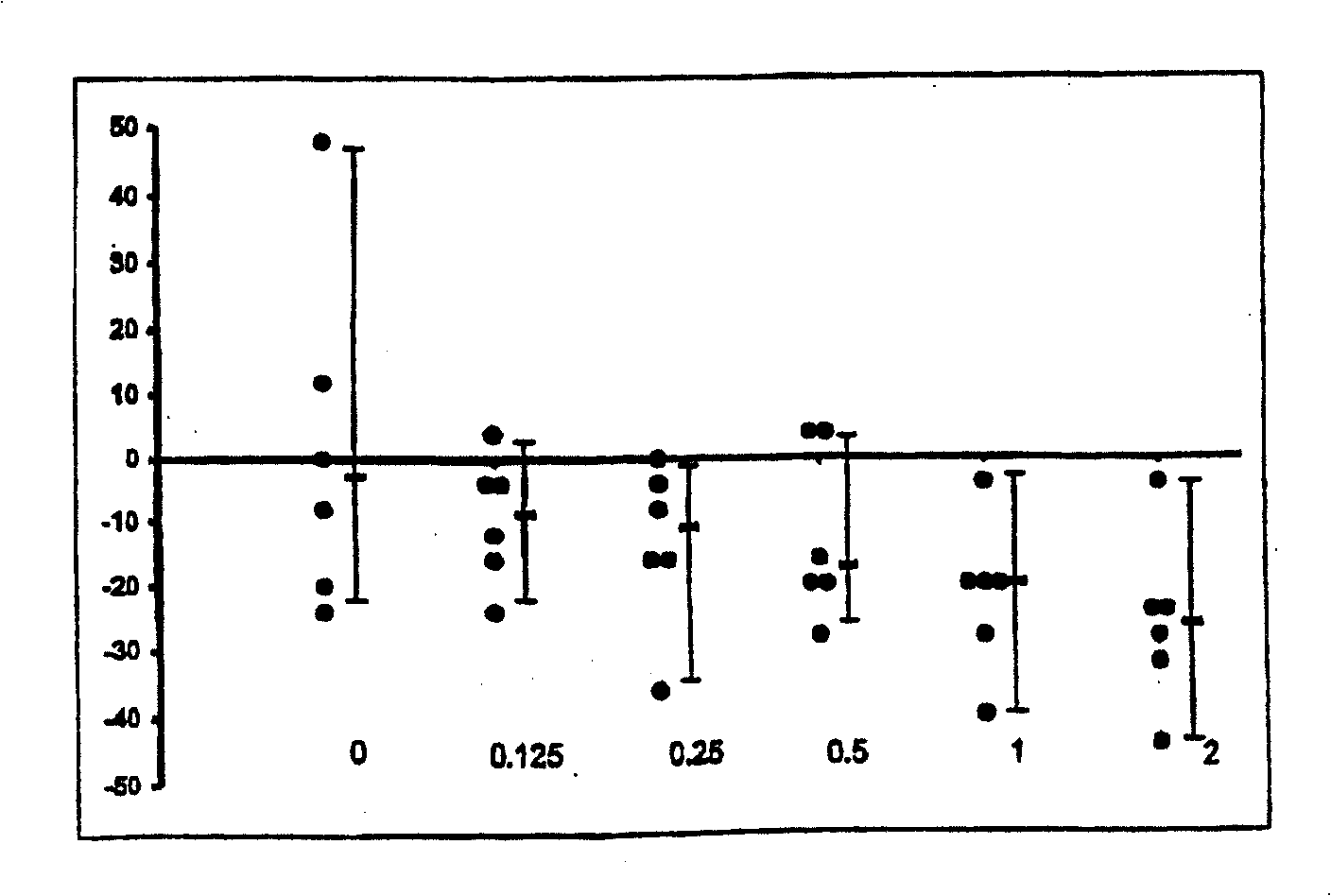
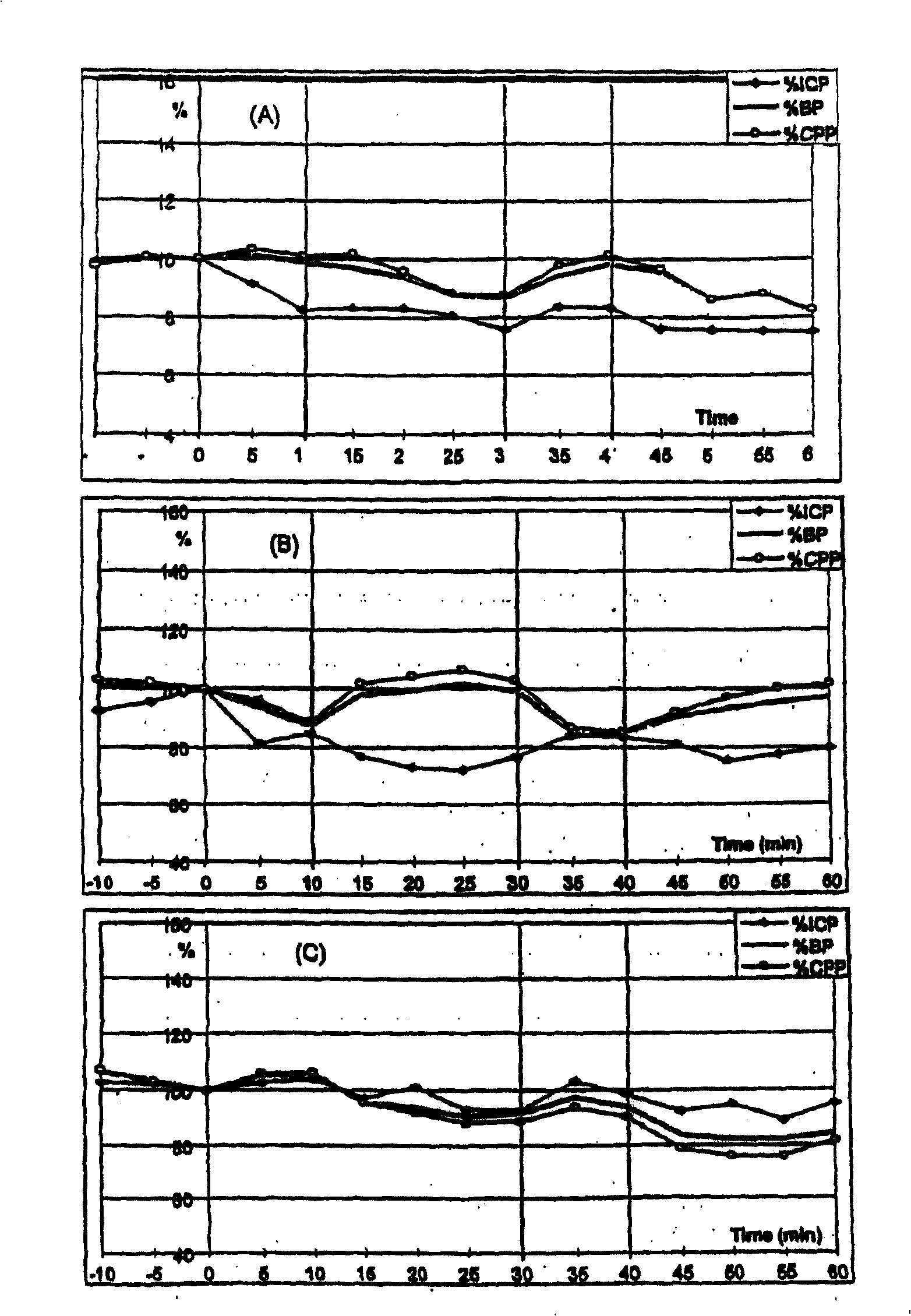
The use of substituted tetracyclic imidazole derivatives as anti-histaminics
一种颅内压、用途的技术,应用在含有效成分的医用配制品、退热药、抗炎剂等方向
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A1
[0105] a) Preparation of Intermediate 1
[0106]
[0107] Use dry glassware. A mixture of (methoxymethyl)triphenylphosphine chloride (0.35mol) and THF p.a. (molecular sieves) (21) was 2 Under air flow, stir at -50°C. BuLi2.5M / hexane (0.35mol) was added dropwise and the resulting mixture was stirred at -25°C for 30 minutes. At -25°C, a THF solution of 1,2-bis(benzyl)-4-piperidone (0.35 mol) was added dropwise. The resulting mixture was allowed to warm to room temperature, then stirred overnight at room temperature, and the mixture was decomposed with water. The organic solvent was evaporated, and the resulting aqueous concentrate was treated with CH 2 Cl 2 extraction. The organic layer was separated and dried (MgSO 4 ), filtered and evaporated to remove the solvent. The resulting residue was purified by silica gel column chromatography (eluent: CH 2 Cl 2 / CH 3 OH 97.5 / 2.5). The purified fractions were collected and the solvent was distilled off. Obtained: 121 ...
Embodiment A2
[0121] a) Preparation of Intermediate 6
[0122]
[0123]Use dry glassware. A mixture of DIPA (0.22mol) and THF p.a. (pre-dried with molecular sieves) (1400ml) in N 2 Stir at -70°C under airflow. BuLi2.5M (0.185 mol) was added dropwise and the resulting mixture was stirred at -70°C for 15 minutes. A solution of 1-(benzyl)-1H-benzimidazole (0.185 mol) dissolved in THF was added dropwise at -70°C, and the resulting mixture was stirred at -70°C for 1 hour. A solution of Intermediate 2 (0.185 mol) dissolved in THF was added dropwise at -70°C. The resulting mixture was stirred at -70°C for 1 hour, then the temperature was slowly raised to room temperature, stirred at room temperature overnight, and the mixture was decomposed with water. The organic solvent was evaporated. Aqueous concentrate with CH 2 Cl 2 extraction. The organic layer was separated and dried (MgSO 4 ), filtered and evaporated to remove the solvent. The resulting residue was purified by silica gel co...
Embodiment A3
[0133] a) Preparation of Intermediate 10
[0134]
[0135] A mixture of DIPA (0.1mol) and THF (100ml) was placed under N 2 Stirring under air flow, the mixture was cooled to -70°C and BuLi 2.5M / hexane (40ml) was added portionwise. The temperature was raised to -30°C while stirring for 10 minutes. The resulting mixture was cooled to -70°C. At this temperature, a solution of 1-(phenethyl)-1H-benzimidazole (0.1 mol) in THF (50 ml) was added dropwise, and the resulting mixture was stirred at -70°C for 2 hours. Ethyl 4-formyl-1-piperidinecarboxylate (0.1 mol) was added dropwise and the resulting mixture was stirred at -70°C for 30 minutes. The mixture was allowed to warm to room temperature and stirring was continued for 30 minutes. The mixture was decomposed with water and evaporated. The resulting residue was stirred in water with CH 2 Cl 2 Extract this mixture. The organic layer was separated and dried (MgSO 4 ), filtered and evaporated to remove the solvent. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com