Method for synthesizing Drospirenone
A trospironone and condensation technology is applied in the field of synthesizing the steroid progesterone trospirenone, which can solve the problems of low yield, poor specificity, low yield and the like of the 7-position hydroxyl group, and achieves large-scale industrial production value and low cost. , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
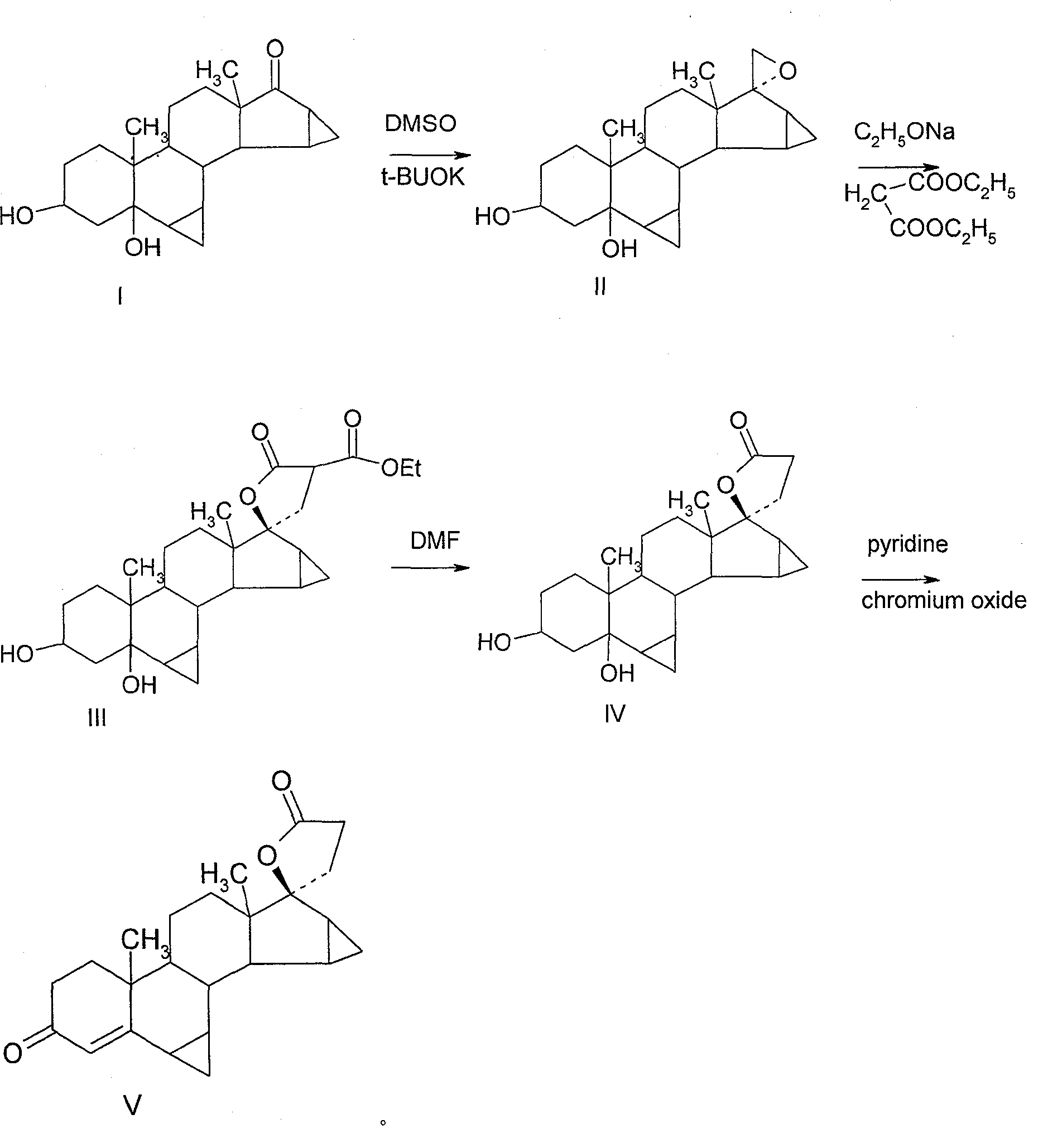
[0031] Example 1 3β, 5-dihydroxy-6β, 7β, 15β, 16β-dimethylene-5β-androst-17-epoxy
[0032] Operation: Heat 30ml of DMSO, 20ml of THF, and 5g of potassium tert-butoxide to 60°C for 0.5 hours, cool to below 0°C, quickly add 8g of bromotrimethylsulfide, stir at 0°C for 30 minutes, add S-10, and React at 0°C for one hour, TLC detects that the reaction is complete, analyze with ice water, filter, and dry to obtain 4.95 g of solid, and the plate layer shows that there are basically no impurities. TLC: cyclohexane: ethyl acetate = 1:2
Embodiment 2
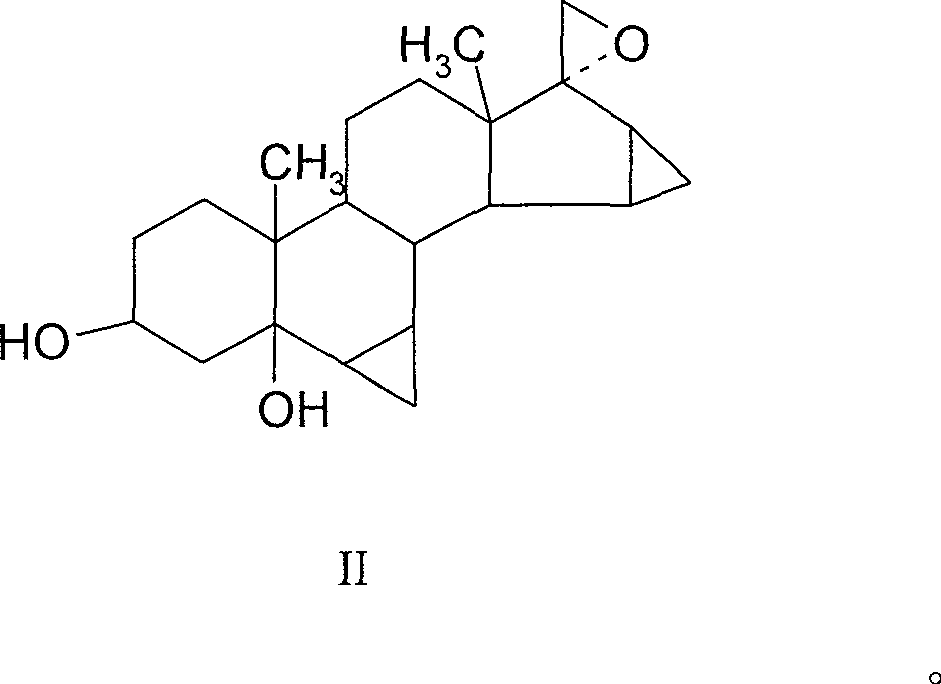
[0033] Example 2 3β, 5-dihydroxy-6β, 7β, 15β, 16β-dimethylene-5β-androsta-21,17-carboxylide-21-ethyl carboxylate
[0034] Operation: Dissolve 3.6g of sodium ethoxide in 30ml of absolute ethanol, heat to 30-35 degrees, add 9ml of diethyl malonate, the internal temperature rises to 35-40 degrees, continue stirring for 10 minutes, put in 3g of raw materials, heat After reflux for 3 hours, the layer detection reaction was completed, cooled to room temperature, and water analysis. Filter to obtain a solid, and dry at room temperature 3.45g (do not heat and dry). The plies show slight impurities on top.
[0035] TLC cyclohexane: ethyl acetate = 1:2
Embodiment 3
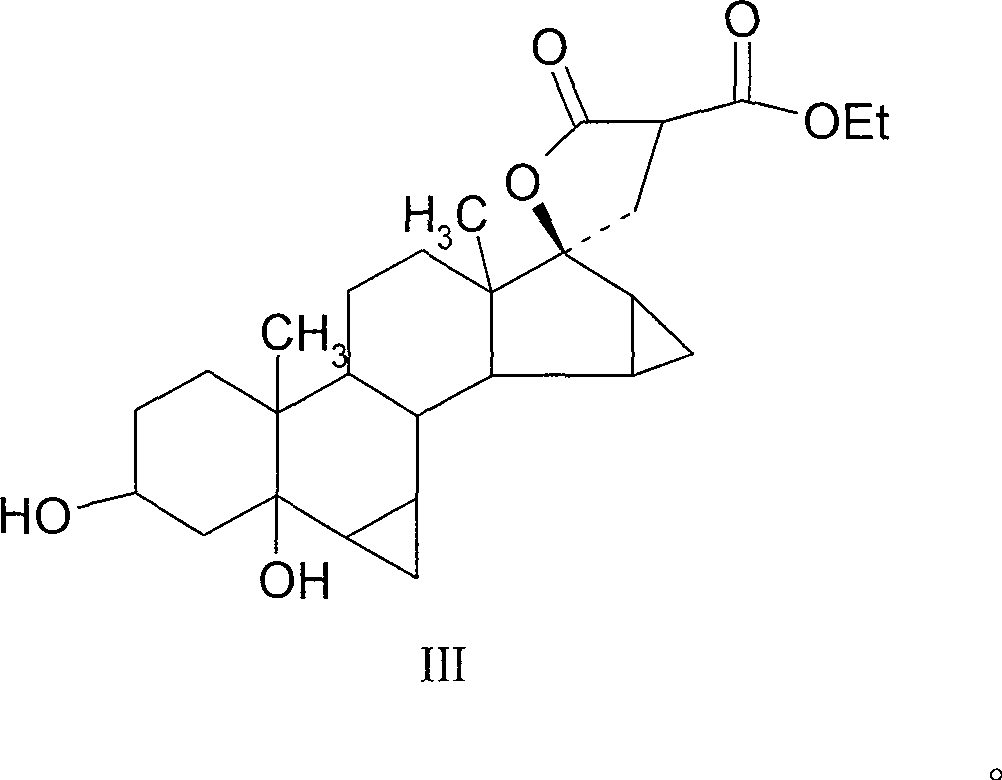
[0036] Example 3 3β, 5-dihydroxy-6β, 7β, 15β, 16β-dimethylene-5β-androsta-21,17-carboxylate
[0037] Operation: Dissolve 11.2g of the raw material in 67ml of DMF under the protection of argon, add water, heat and reflux for 4 hours, TLC detects that the reaction is complete, cool to room temperature, analyze with saturated saline, filter, place and dry at room temperature to obtain 9.2g, and proceed directly Oxidized, the ply shows slight impurities.
[0038] TLC cyclohexane: ethyl acetate = 1:2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com