Method for preparing luminescent material with long persistence of Nano strontium aluminate
A technology of luminescent materials and nano-aluminum, which is applied in the direction of luminescent materials, chemical instruments and methods, and can solve problems such as agglomeration of luminescent powders and incomplete particle development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
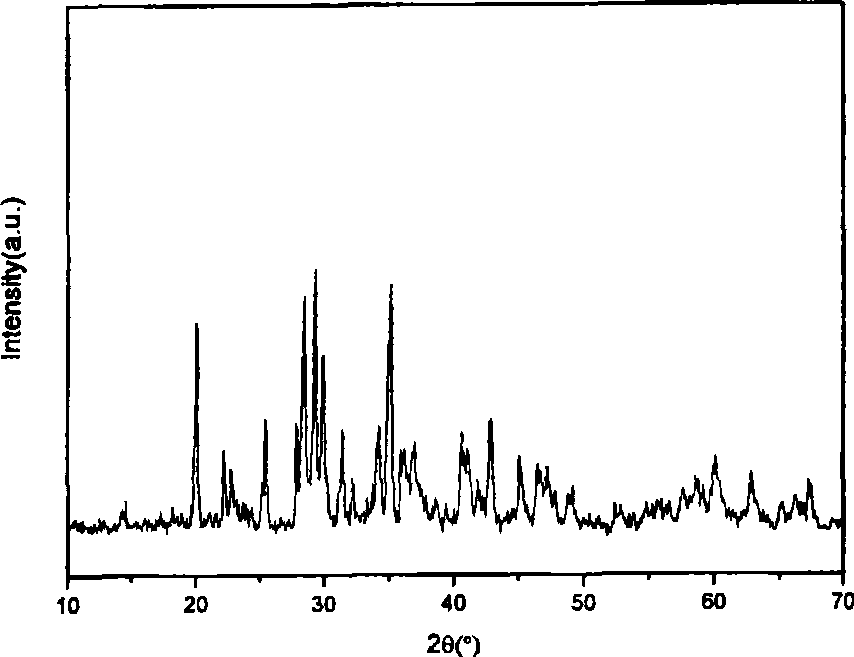
[0030] Prepare 1mol / L Al(NO 3 ) 3 and Sr(NO 3 ) 2 solution, the Eu 2 o 3 and Dy 2 o 3Dissolve in nitric acid solution respectively, adjust the concentration to 0.1mol / L, precipitant NH 4 ) 2 CO 3 The concentration of the solution is 1.0 mol / L. According to chemical formula Sr 3.88 Al 14 o 25 :Eu 0.04 , Dy 0.08 Al(NO 3 ) 3 , Sr(NO 3 ) 2 , Eu(NO 3 ) 3 and Dy(NO 3 ) 3 The mixed solution of each concentration is 0.7M, 0.2M, 0.004M and 0.008M respectively. In a water bath at 60°C, slowly drop (NH 4 ) 2 CO 3 Mix the solution with nitrate (mode (1)), and stir rapidly for 3h.
[0031] Put 25ml of the suspension of the resulting product precursor into a 30ml hydrothermal kettle, treat it at 140°C for 12h, wash the treated suspension with water and alcohol for 3 to 4 times, and dry it in an air oven at 80°C for 24 hours , take out and grind, pass through a 200-mesh sieve and then put in a weak reducing atmosphere (96%N 2 +4%H 2 ), calcined in a high-temperat...
Embodiment 2
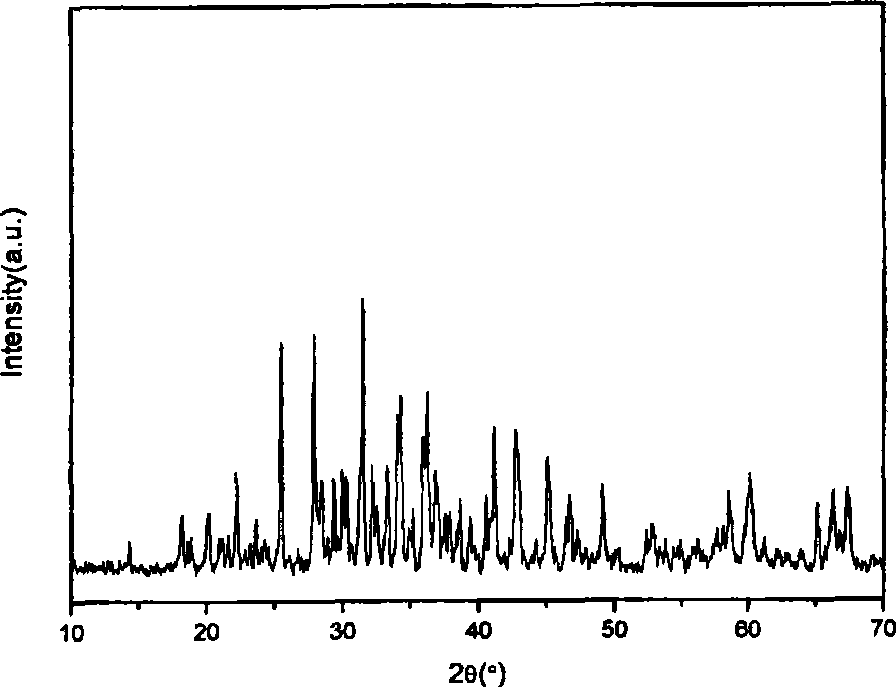
[0034] Prepare 1mol / L Al(NO 3 ) 3 and Sr(NO 3 ) 2 solution, the Eu 2 o 3 and Dy 2 o 3 Dissolve in nitric acid solution respectively, and adjust the concentration to 0.1mol / L. According to chemical formula Sr 3.88 Al 14 o 25 :Eu 0.04 , Dy 0.08 Al(NO 3 ) 3 , Sr(NO 3 ) 2 , Eu(NO 3 ) 3 and Dy(NO 3 ) 3 The mixed solution of each concentration is 0.7M, 0.2M, 0.004M and 0.008M respectively. In a water bath at 60°C, slowly drop (NH 4 ) 2 CO 3 Mix solution with nitrate (mode (1)), stir rapidly for 3h, wherein, (NH 4 ) 2 CO 3 The solution was used in excess of 20% required for complete precipitation of the various ions at a concentration of 1.5M.
[0035] Put 25ml of the suspension of the product precursor produced by the reaction into a 30ml hydrothermal kettle, treat at 200°C for 4h, wash the treated suspension with water and alcohol for 3 to 4 times, and dry in an air oven at 80°C for 24 hours , take out and grind, pass through a 200-mesh sieve and then put...
Embodiment 3
[0038] Prepare 1mol / L Al respectively 2 (SO 4 ) 3 and SrSO 4 solution, the Eu 2 o 3 and Dy 2 o 3 Dissolve in sulfuric acid solution respectively, and adjust the concentration to 0.1mol / L. According to chemical formula Sr 3.88 Al 14 o 25 :Eu 0.04 , Dy 0.08 Al(NO 3 ) 3 , SrSO 4 、Eu 2 (SO 4 ) 3 and Dy 2 (SO 4 ) 3 The mixed solution of each concentration is 0.7M, 0.2M, 0.004M and 0.008M respectively. In a water bath at 60°C, slowly drop (NH 4 ) 2 CO 3 (mode (2)), rapid stirring 3h, wherein, (NH 4 ) 2 CO 3 The solution was used in excess of 20% required for complete precipitation of the various ions at a concentration of 1.5M.
[0039] Put 25ml of the resulting product precursor suspension into a 30ml hydrothermal kettle, treat it at 200°C for 4h, wash the hydroheated suspension with water and alcohol for 3 to 4 times, and dry it in an air oven at 80°C for 24 hours , take out the grind and mix with 2%wt H 3 BO 3 , passed through a 200-mesh sieve and th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com