Steroid saponin compound and use in preparation antineoplastic
A technology of steroidal saponins and compounds, which is applied in the field of steroidal saponins and their new applications in the preparation of antitumor drugs, and can solve the problems of compound biological activity reports and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
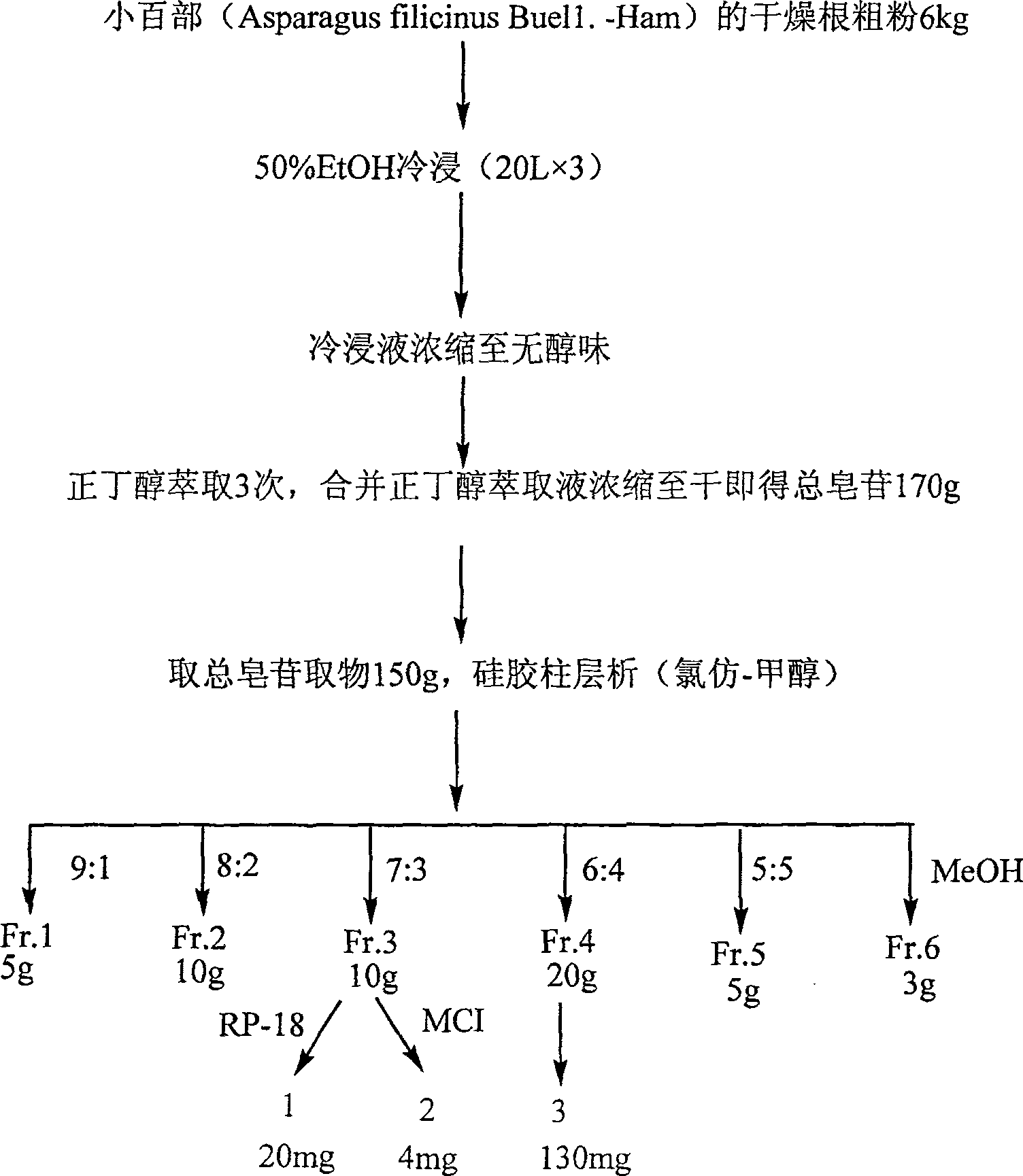
[0023] Example 1 Preparation of total saponins
[0024] Take 6 kg of rhizome powder of Lily chinensis, soak in ethanol at room temperature (20L×3 times), combine the extracts and concentrate until there is no alcohol smell, add water to dilute the extracts to 2L, extract with n-butanol (2L×3 times), combine and normal The butanol extract was concentrated to dryness to obtain 170 g of total saponins. Take 150g of total saponins and apply dry method to carry out silica gel column chromatography, and use chloroform, chloroform-methanol, methanol gradient elution, the specific steps are as follows:
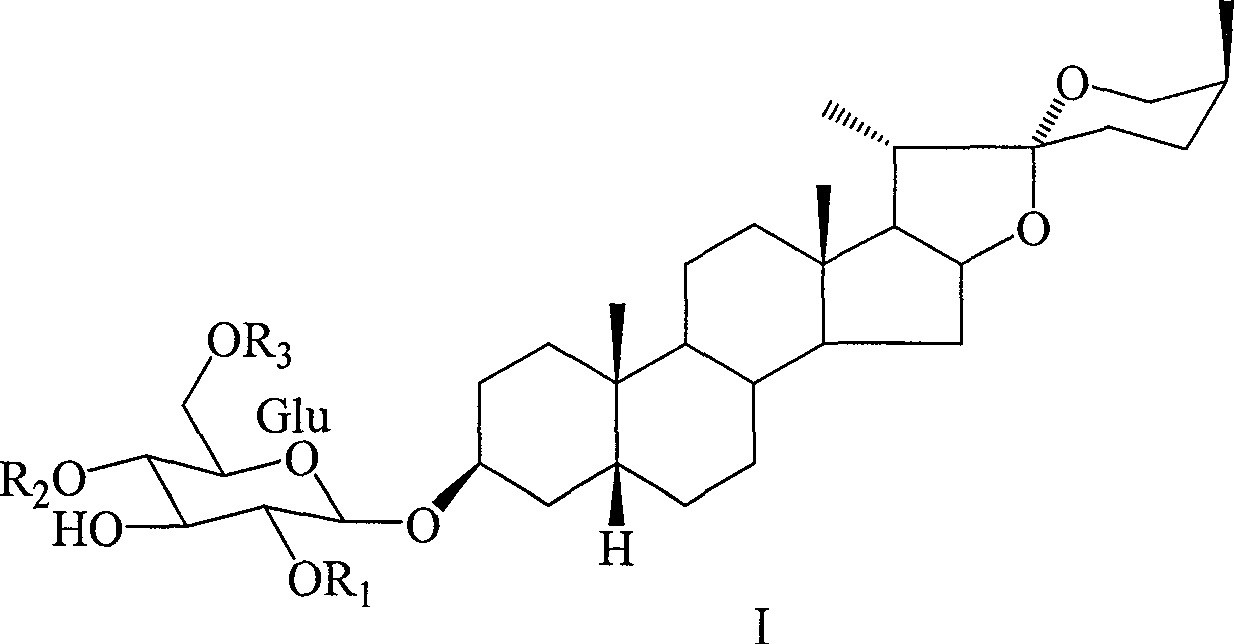
[0025] 1. The obtained fractions were eluted with chloroform-methanol (7:3), repeated silica gel column chromatography with chloroform-methanol-water (4:1:0.2), and then passed through RP-18 column (60-90% methanol) to obtain white Amorphous powder 20mg, that is, paretia saponin A (1). Using spectroscopy and chemical analysis, its structure was determined to be smilagenin-3-O-[β-D-x...
Embodiment 2
[0028] Embodiment 2 in vitro antitumor test (SRB method)
[0029] Two kinds of tumor cell strains of A549 and MCF-7 are contained in the T-25 flask of the 10% calf serum RPMI1640 culture fluid 4ml of 25mM HEPES, 0.2% (w / v) sodium bicarbonate and 100 μ g / ml kanamycin, 37°C, 5% CO 2 cultivated under conditions. The cell suspension digested by trypsin was added to a 96-well plate, and the cell concentration was 0.25-1×10 4 / hole. Tumor cells were added different concentrations of components to be tested and cultured at 37°C for 72 hours, fixed with ice-cold 50% trichloroacetic acid and stained with 0.4% (SRB). After the dye was dissolved, the absorbance was measured at 562 nm. The drug concentration EC at the time of half cell growth inhibition 50 Conversion was performed according to dose-effect data. Each experiment was repeated three times, and the difference in absorbance value was less than 5%, EC 50 The difference is less than 30%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com