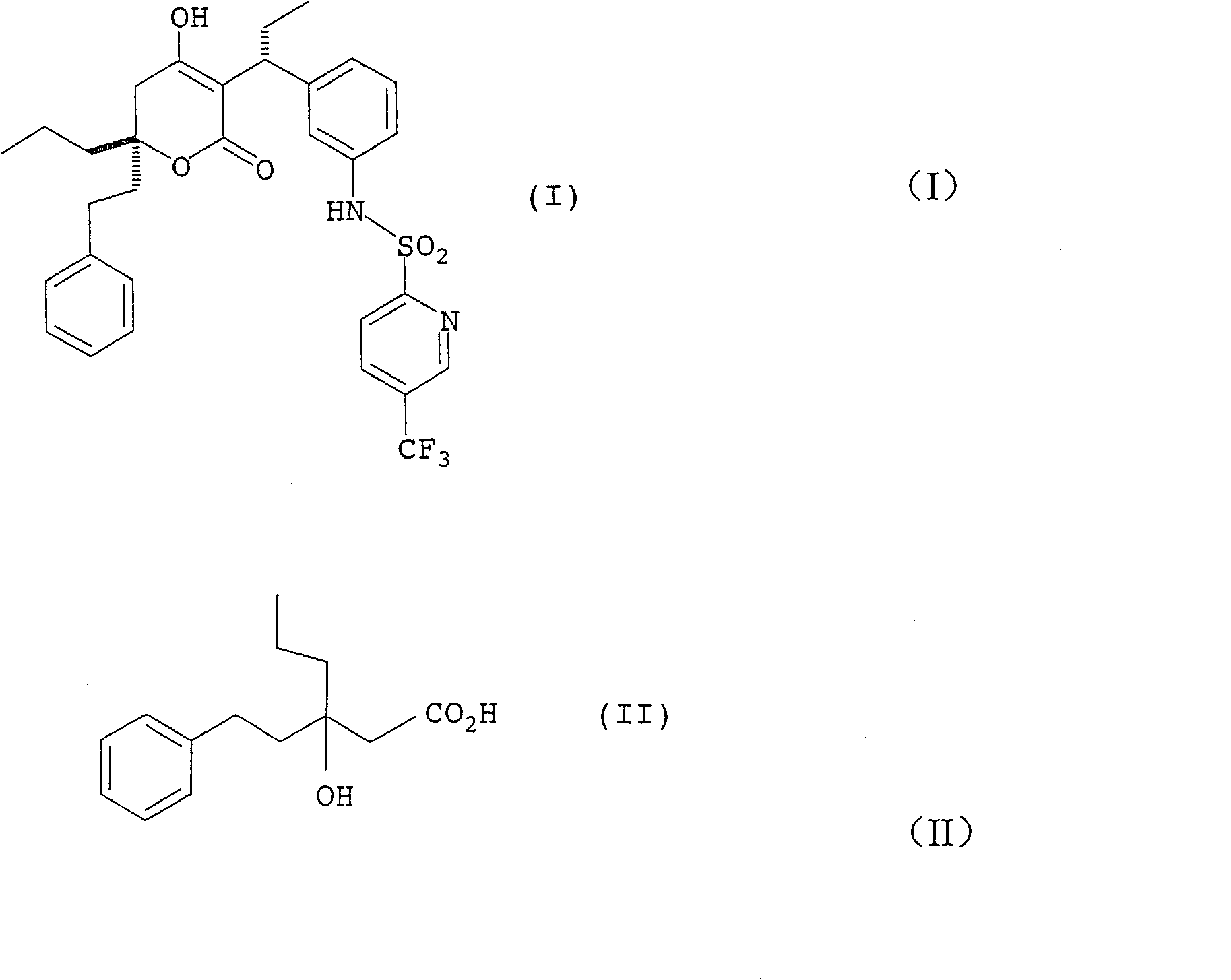
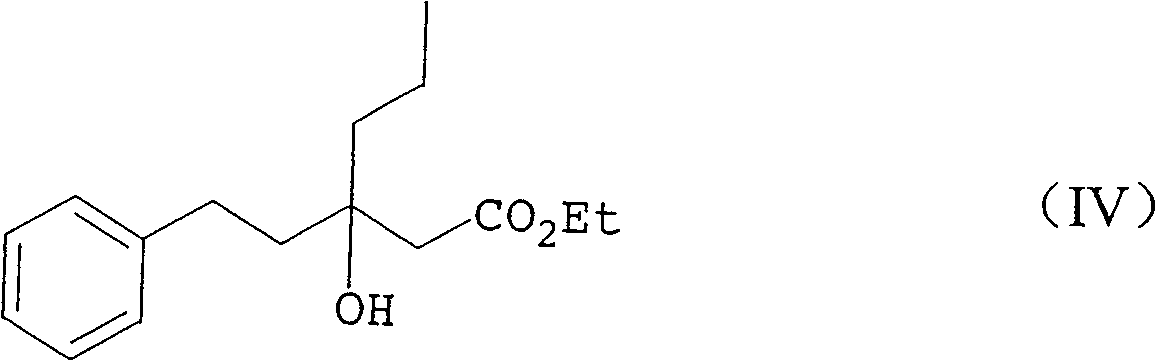
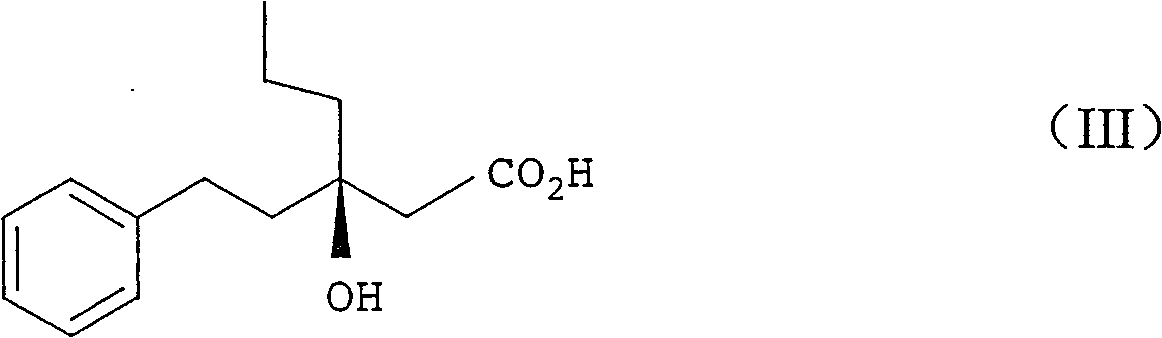Production method of racemiation3-hydroxy-3- (2-phenylethyl) hexanoic acid C1-6 alkyl ester
A production method and technology of phenylethyl are applied in the production field of racemic 3-hydroxy-3-(2-phenylethyl)hexanoic acid C1-6 alkyl ester, which can solve the problem that bromoacetate is expensive And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0296] rac ethyl 3-hydroxy-3-(2-phenylethyl)hexanoate
[0297] Magnesium (37.2 g, 1.53 mol) was suspended in THF (60.5 g) and allyl bromide (1.4 g, 0.01 mol) was added in a fine stream. After raising the temperature from 22°C to 38°C, dimethyldichlorosilane (7.3 g, 0.06 mol), 1-phenyl-3-hexanone ( 180.2 g, 1.02 mol), ethyl chloroacetate (139.1 g, 1.13 mol) and allyl bromide (1.4 g, 0.01 mol) in THF (472.9 g). Further ethyl chloroacetate (41.7 g, 0.34 mol) was added in a fine stream at 30-40°C over 1 hour. The mixture was stirred at 40°C for 1.5 hours, and unreacted magnesium was separated by decantation. The decanted reaction mixture was added to a mixture of 35% hydrochloric acid (151.8g, 1.50mol), ammonium chloride (15.9g, 0.30mol) and water (280.7g) with a fine stream, and the layers were separated at 30-40°C . Concentration of the organic layer gave a residue (342.6 g) (quantitative analysis by HPLC) containing the title compound (215.1 g, yield 79.6%).
[0298] 1 ...
Embodiment 2
[0300] rac ethyl 3-hydroxy-3-(2-phenylethyl)hexanoate
[0301] Magnesium (7.45 g, 0.31 mol) was suspended in THF (12.1 g) and allyl bromide (0.28 g, 0.0023 mol) was added in a fine stream. After raising the temperature from 20°C to 30°C, methyltrichlorosilane (3.39 g, 0.023 mol), 1-phenyl-3-hexanone (40.0 g, 0.227 mol), ethyl chloroacetate (27.81 g, 0.23 mol) and allyl bromide (0.275 g, 0.0023 mol) in THF (94.6 g). Further ethyl chloroacetate (8.34 g, 0.07 mol) was added in a fine stream at 30-40°C over 40 minutes.
[0302] The mixture was stirred at 40° C. for 2 hours, and the title compound in the reaction mixture was analyzed to obtain a reaction mixture (46.74 g, yield 77.9%) containing the title compound (quantitative analysis by HPLC). The NMR data is the same as in Example 1.
Embodiment 3
[0304] rac ethyl 3-hydroxy-3-(2-phenylethyl)hexanoate
[0305] Magnesium (7.45 g, 0.31 mol) was suspended in THF (12.1 g) and allyl bromide (0.28 g, 0.0023 mol) was added in a fine stream. After raising the temperature from 20°C to 30°C, silicon tetrachloride (3.86 g, 0.023 mol), 1-phenyl-3-hexanone (40.0 g , 0.227mol), ethyl chloroacetate (27.81g, 0.23mol) and allyl bromide (0.275g, 0.0023mol) in THF (94.6g). Further ethyl chloroacetate (8.34 g, 0.07 mol) was added in a fine stream at 30-40°C over 40 minutes.
[0306] The mixture was stirred at 40° C. for 2 hours, and the title compound in the reaction mixture was analyzed to obtain a reaction mixture (46.76 g, yield 77.9%) containing the title compound (quantitative analysis by HPLC). The NMR data is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com