Medicinal composition for treating chronic kidney region fibrosis and its preparation method
A technology of renal interstitial fibrosis and composition, which is applied in the field of pharmaceutical composition for the treatment of chronic renal interstitial fibrosis and its preparation, and can solve problems such as unclear pathogenesis, end-stage renal failure, and no treatment methods, etc. Achieve the effect of reversing or alleviating renal interstitial fibrosis and improving renal function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0036] Experimental Example 1 Yishen Ruanjian Powder Containing Serum Antagonizes the Effect of Aristolochic Acid on Human Renal Interstitial Fibroblasts
[0037] Male SD rats, 2 months old, with a body weight of 270-290g, fasted for 16 hours, without anesthesia, were gavaged with Yishenruanjian powder water decoction (11ml / kg body weight for each gavage, which is equivalent to 60kg 10 times the body weight of a person), blood was collected from the inner canthus vein of rats at 1h after the single gavage and 0.5h, 1h and 1.5h after the second gavage (interval 2h) respectively (6 rats were taken at each time point ), the serum was collected after centrifugation, and the sera of 6 rats were mixed, sterilized by filtration, and inactivated at 56° C. for 30 min. This was the drug-containing serum for the experiment. Rat normal serum was obtained in the same way. High performance liquid chromatography (high performance liquid chromatography, HPLC) was used to determine the conten...
experiment example 2
[0051] Experimental Example 2 Yishen Ruanjian Powder Containing Serum Antagonizes the Effect of Aristolochic Acid on Human Proximal Renal Tubular Epithelial Cells
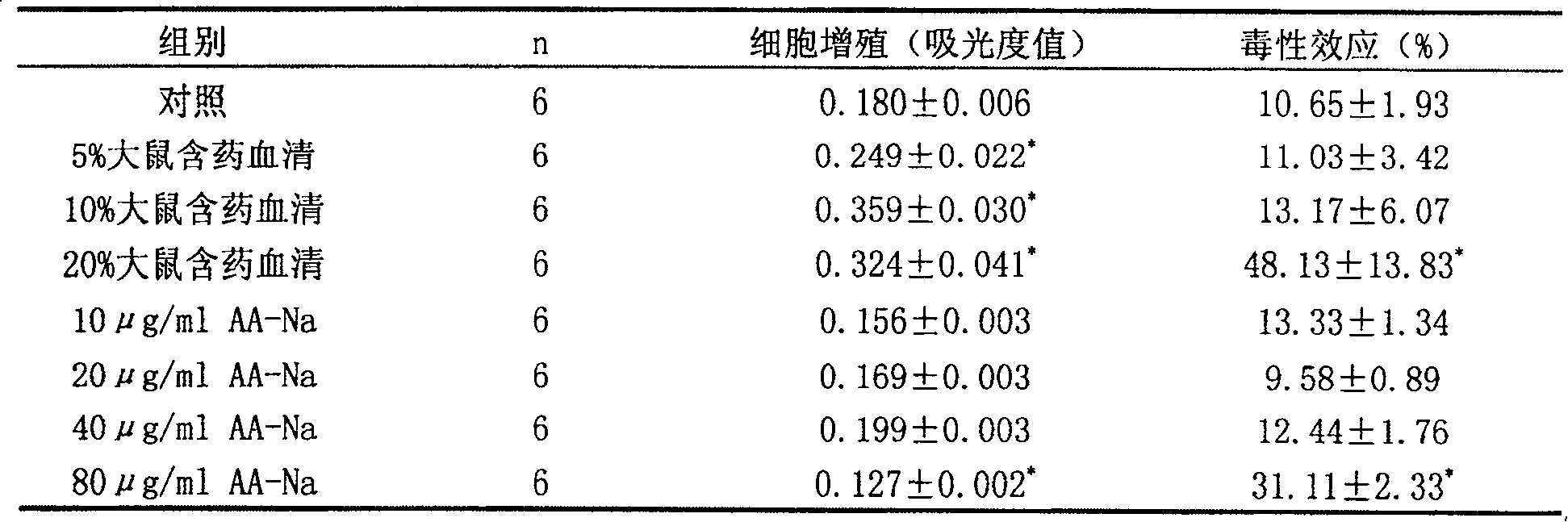
[0052] (1) Detection of rat serum and AA-Na on HKC cell proliferation and toxicity
[0053] The results of the LDH release experiment showed that when the drug-containing serum content of rats was 5% and 10%, there was no obvious toxic effect on HKC cells (P>0.05), while the drug-containing serum of rats had a killing effect on HKC when the content was 20% (P -1 No obvious toxic effect on HKC (P>0.05), while 80mg·L -1At this time, AA-Na can kill HKC (P-1 . For this reason, add AA-Na40mg·L to 10% rat serum containing medicine -1 The cytotoxicity test was carried out again, and there was no significant difference in the release rate of LDH compared with the control group (P>0.05) (Table 1). The above results indicated that the co-incubation of AA-Na with the content of this experiment and the drug-containing serum...
experiment example 3
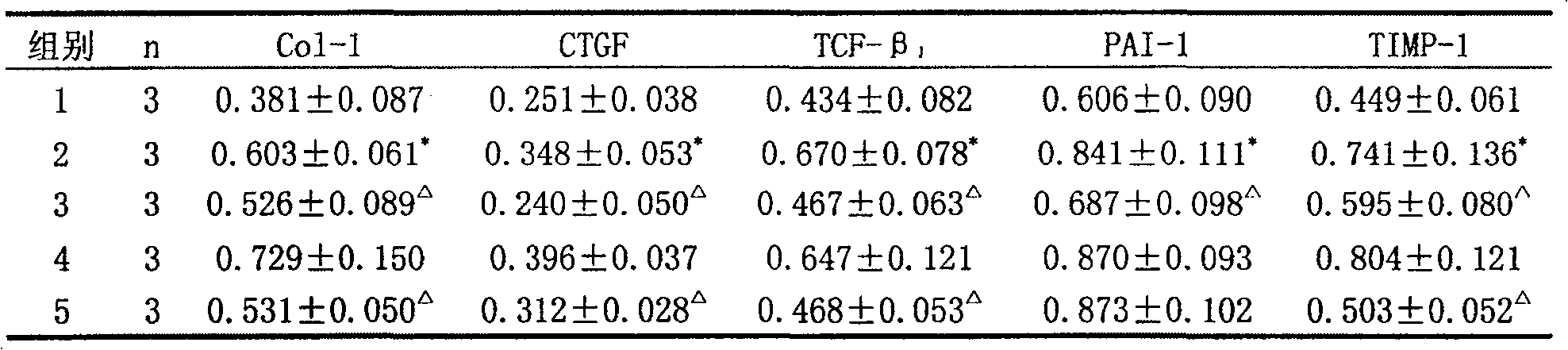
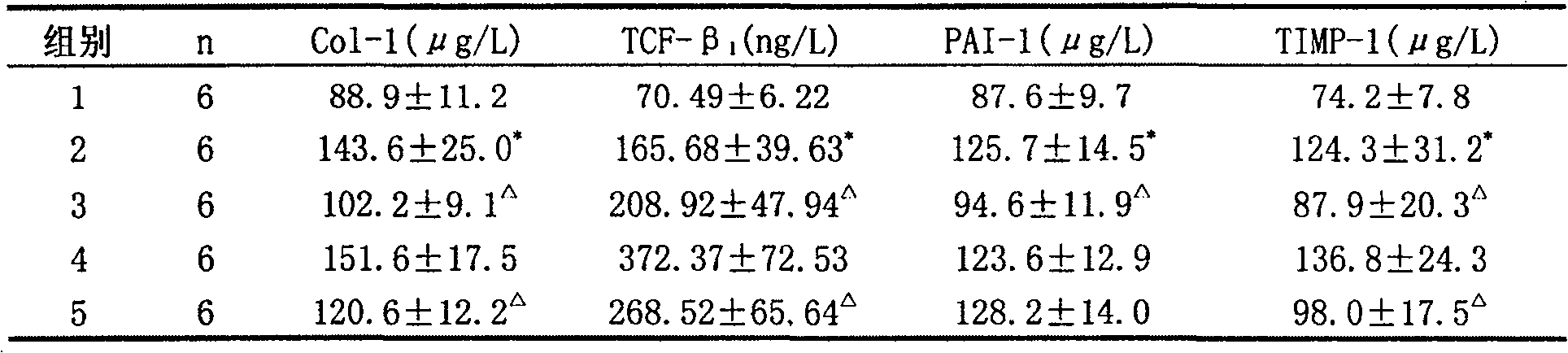
[0069] Experimental Example 3 Protective Effect of Yishen Ruanjian Powder on Renal Interstitial Fibrosis in Chronic Aristolochic Acid Nephropathy Rat Model
[0070] Eighteen male SD rats were divided into 3 groups with 6 rats in each group. Control group: intragastric administration with tap water every morning; model group: intragastric administration with Guan Mutong extract aqueous solution in the morning, the method was the same as that in the literature; Oral gavage in the afternoon of the first day; a total of 16 weeks.
[0071] (1) Comparison of body weight of rats in each group at different time points
[0072] Before the experiment, there was no significant difference in the body weight of the rats in each group (data omitted), and the body weight of the rats in the model group and the Chinese medicine group were significantly lower than that of the control group after administration; the rats in the Chinese medicine group and the model group had no significant diffe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com