Hydrochloric acid dronedarone medicinal compositions for oral use and method for preparing the same
A technology of dronedarone hydrochloride and a composition, applied in the field of solid pharmaceutical compositions, can solve the problems of complex manufacturing process, poor stability and the like, and achieve the effect of solving the problem of dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
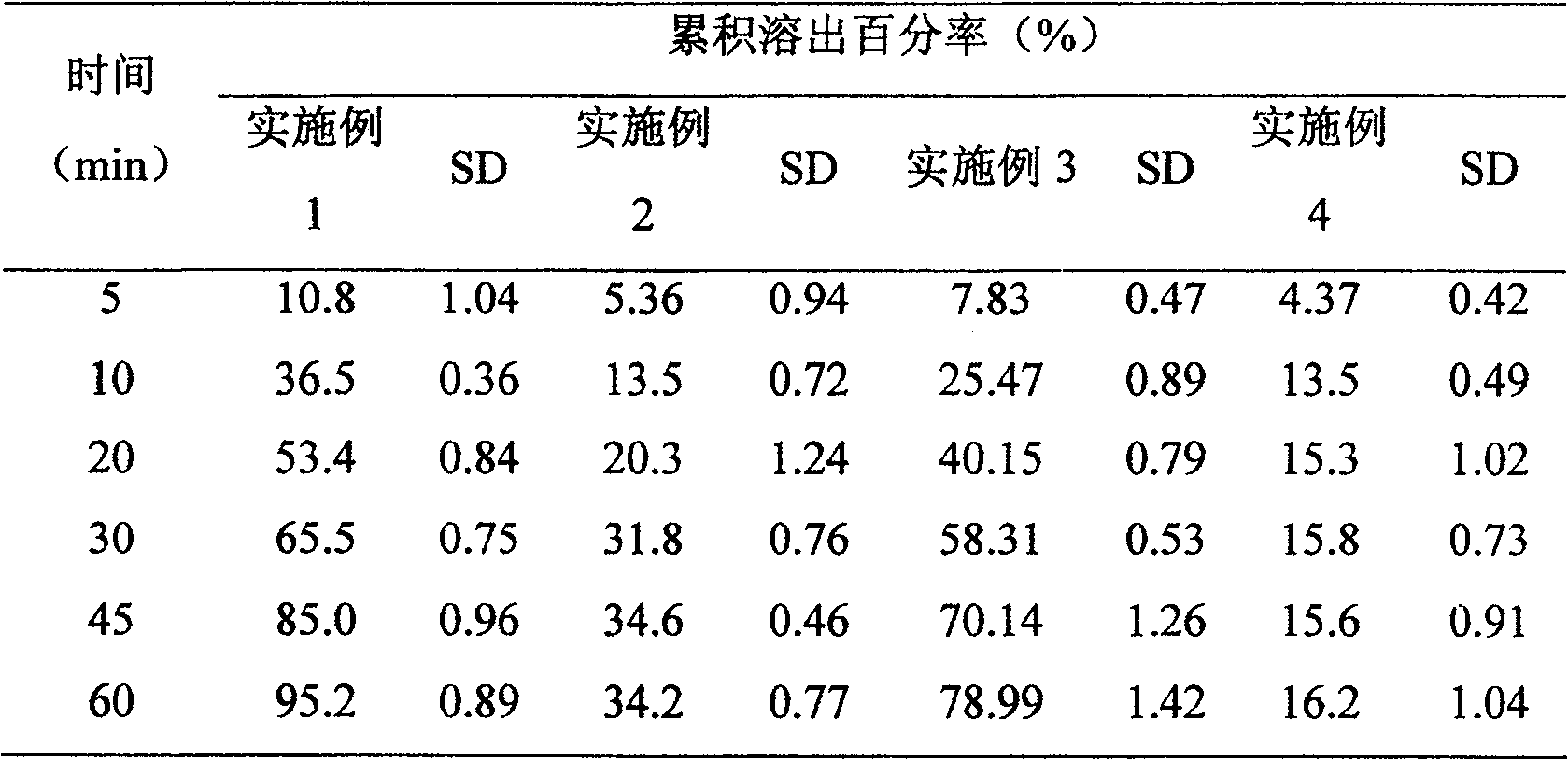
Embodiment 1
[0041] (1) The dronedarone hydrochloride is pulverized by a fluidized bed air flow to obtain micronized dronedarone hydrochloride with an average particle size of less than 15 nm;
[0042] (2) Sodium lauryl sulfate and polyvinylpyrrolidone k30 are completely dissolved in water, and then the micronized dronedarone hydrochloride obtained in (1) is suspended therein to obtain a suspension;
[0043] (3) Suspending the lactose in a fluidized bed granulator, spraying the suspension obtained in (2) into the lactose for granulation to obtain granules;
[0044] (4) Add microcrystalline cellulose PH101 and magnesium stearate to the granules obtained in step (3), and further compress them into tablets.
Embodiment 2
[0047] (1) Crushing dronedarone hydrochloride with a fluidized bed air flow to obtain micronized dronedarone hydrochloride with an average particle size greater than 15 μm and less than 50 μm;
[0048] (2) Sodium lauryl sulfate and polyvinylpyrrolidone k30 are completely dissolved in water, and then the micronized dronedarone hydrochloride obtained in (1) is suspended therein to obtain a suspension;
[0049] (3) suspending the lactose in a fluidized bed granulator, spraying the suspension obtained in (2) into the lactose for granulation to obtain granules;
[0050](4) Add microcrystalline cellulose PH101 and magnesium stearate to the granules obtained in step (3), and further compress them into tablets.
Embodiment 3
[0053] (1) The dronedarone hydrochloride is pulverized by a fluidized bed air flow to obtain micronized dronedarone hydrochloride with an average particle size of less than 15 nm;
[0054] (2) Dissolving polyvinylpyrrolidone k30 completely in water, and then suspending the micronized dronedarone hydrochloride obtained in (1) to obtain a suspension;
[0055] (3) Suspending the lactose in a fluidized bed granulator, spraying the suspension obtained in (2) into the lactose for granulation to obtain granules;
[0056] (4) Add microcrystalline cellulose PH101 and magnesium stearate to the granules obtained in step (3), and further compress them into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com