Inorganic-organic composite material with photochromic feature and preparation process thereof
A composite material and photochromic technology, which is applied in the field of hydrotalcite, can solve problems such as the inability to confirm the cause of discoloration, and achieve the effects of pollution-free preparation, easy reaction, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Step A: 35.69g (0.12mol) of solid Zn(NO 3 ) 2 ·6H 2 O and 22.51g (0.06mol) of solid Al(NO 3 ) 3 9H 2 O was dissolved in 100ml of water.
[0034] Step B: Dissolve 12g NaOH in 100ml water to prepare a 3.0M alkali solution.
[0035] Step C: While vigorously stirring, slowly add the alkali solution to the salt solution dropwise, adjust the pH=6 to complete the dropwise addition, crystallize at 70°C for 24h, filter and wash until the pH value is about 7, and dry at 70°C for 24h to obtain zinc Aluminum nitrate-type hydrotalcite material.
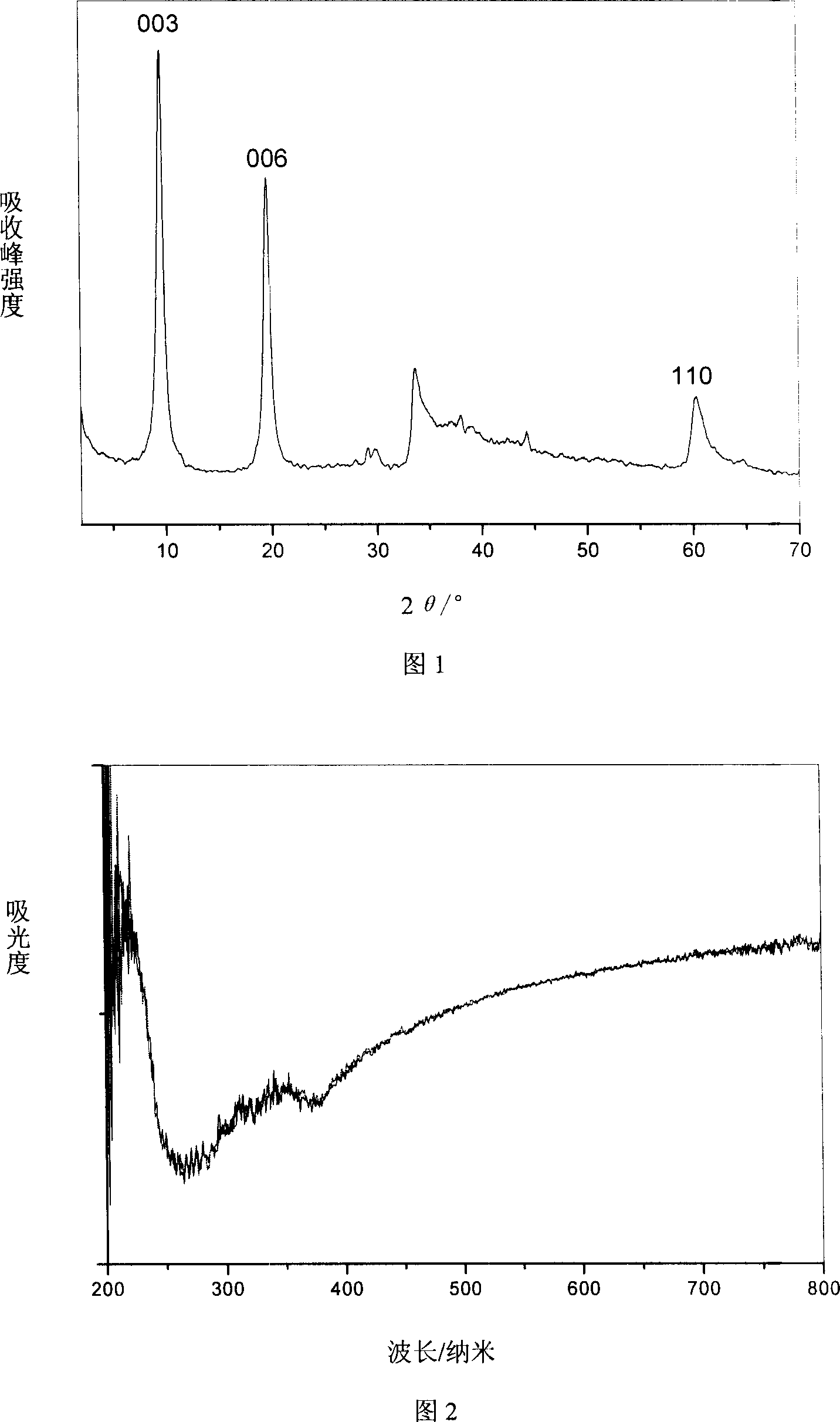
[0036] From the XRD spectrum (Figure 1), IR spectrum and elemental analysis, it can be known that the interlayer anions of the obtained hydrotalcite are all nitrate, which is a LDHs material with a single crystal phase and a consistent structure.
[0037] The LDHs were irradiated under a 500W high-pressure xenon lamp for 20 minutes, and the color did not change (Figure 2).
Embodiment 2
[0039] Step A: A certain amount of m-nitrobenzoic acid (3.35 g) was dissolved in 100 ml of deionized water.
[0040] Step B: Dissolve 3g of NaOH in 80ml of deionized water and add dropwise to solution A until pH=6.
[0041] Step C: add an appropriate amount of zinc-aluminum nitrate-type hydrotalcite precursor prepared in Example 1 to the above mixed solution, stir vigorously, crystallize at 70°C for 24h, filter, wash until the pH value is about 7, and dry at 70°C for 24h to obtain Zn-Al hydrotalcite materials intercalated with m-nitrobenzoic acid.
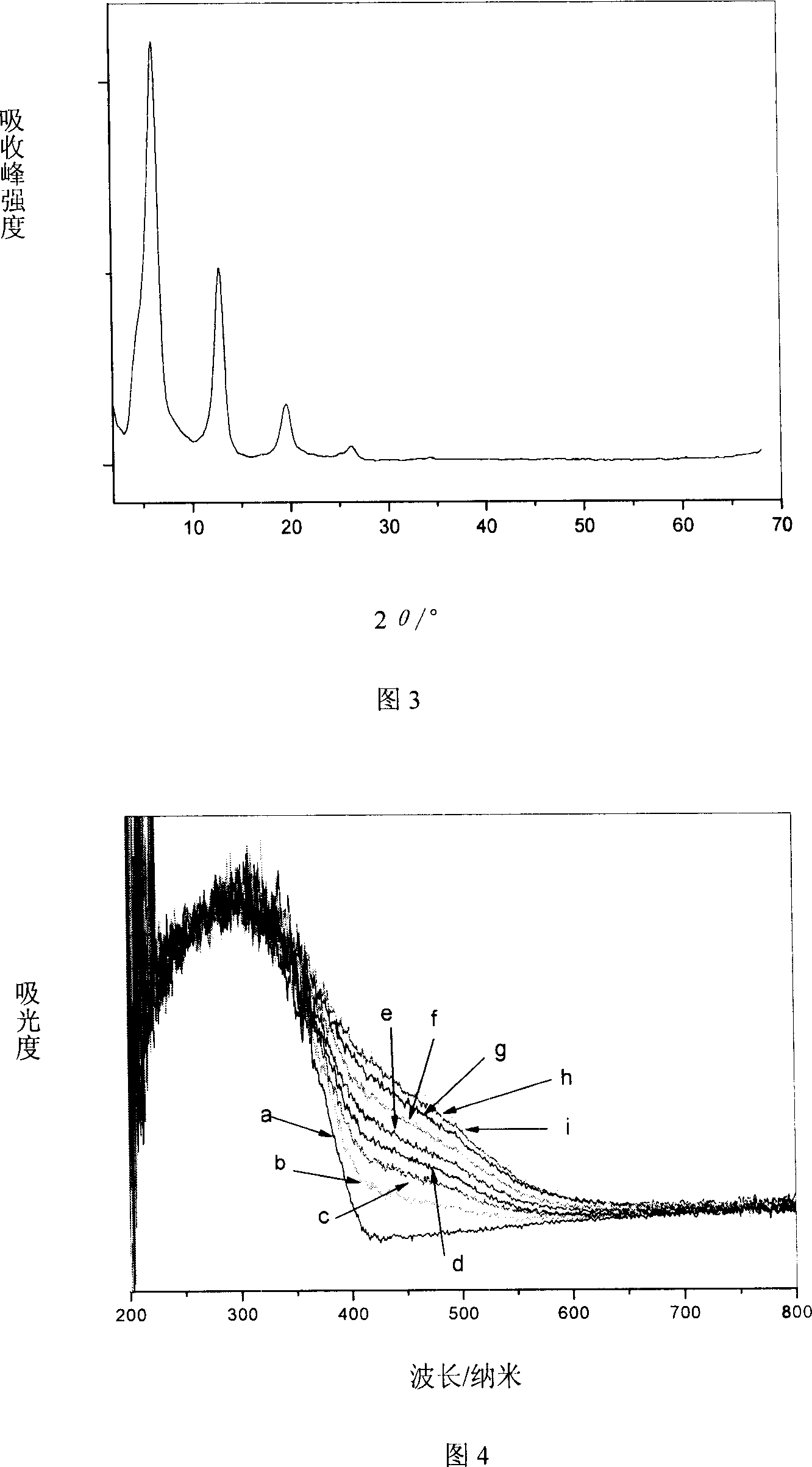
[0042] From the XRD spectrum (Figure 3), IR spectrum and elemental analysis, it can be seen that the interlayer anions of the obtained hydrotalcite are all m-nitrobenzoate, which is a LDHs material with a single crystal phase and a consistent structure.
[0043] The LDHs was irradiated under a 500W high-pressure xenon lamp for 20 minutes, and its color changed from yellow-white to yellow-brown. After heating at 70°C for a period o...
Embodiment 3
[0045] Step A: A certain amount of o-nitrobenzoic acid (3.35 g) was dissolved in 100 ml of deionized water.
[0046] Step B: Dissolve 3g of NaOH in 80ml of deionized water and add dropwise to solution A until pH=6.
[0047] Step C: add an appropriate amount of zinc-aluminum nitrate-type hydrotalcite precursor prepared in Example 1 to the above mixed solution, stir vigorously, crystallize at 70°C for 24h, filter, wash until the pH value is about 7, and dry at 70°C for 24h to obtain Zn-Al hydrotalcite material intercalated with o-nitrobenzoic acid.
[0048] From the XRD spectrum (Figure 3), IR spectrum and elemental analysis, it can be known that the interlayer anions of the obtained hydrotalcite are all o-nitrobenzoate, which is a LDHs material with a single crystal phase and a consistent structure.
[0049] The LDHs was irradiated under a 500W high-pressure xenon lamp for 20 minutes, and its color changed from yellow-white to yellow-brown. After heating at 70°C for a period of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com