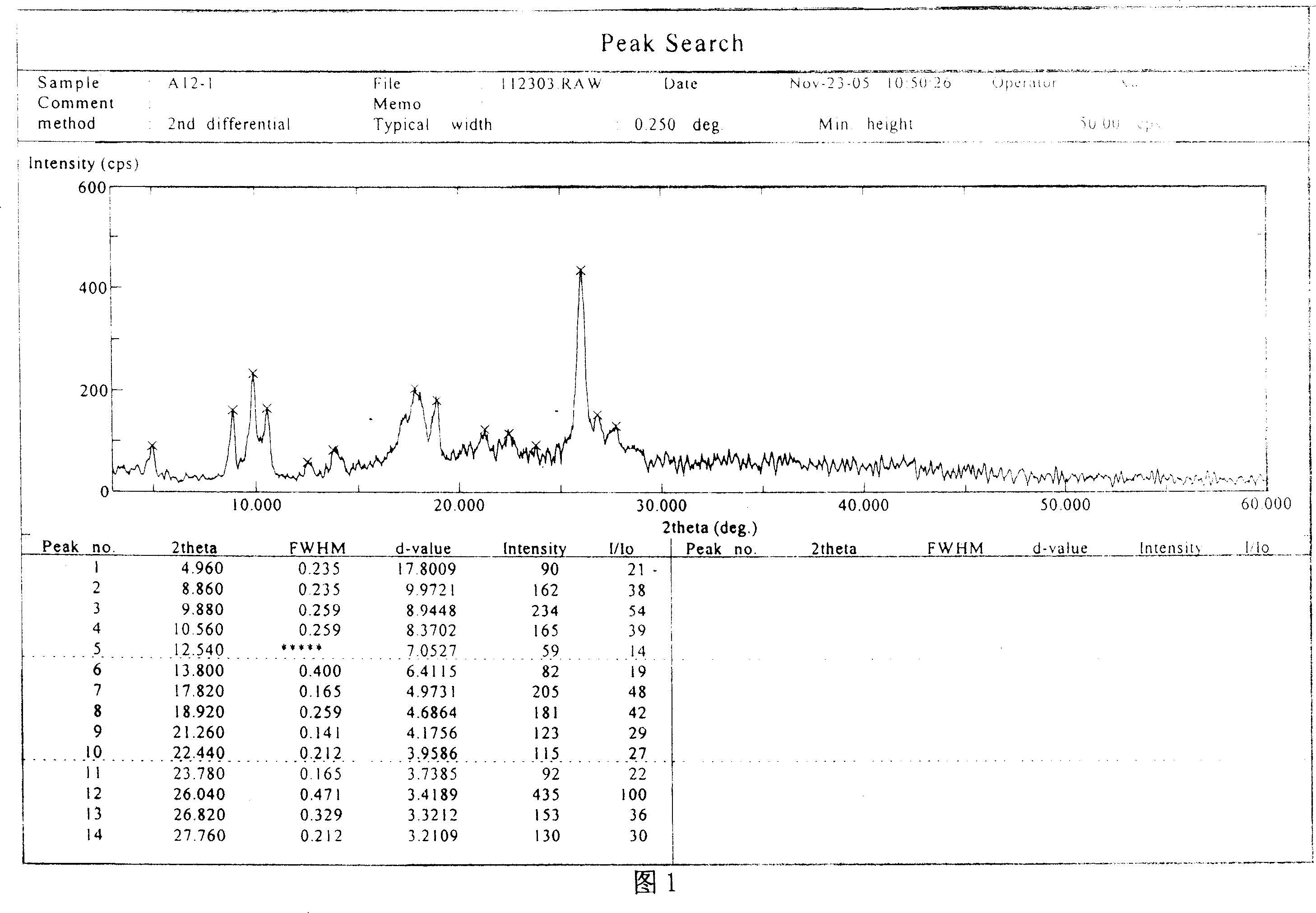
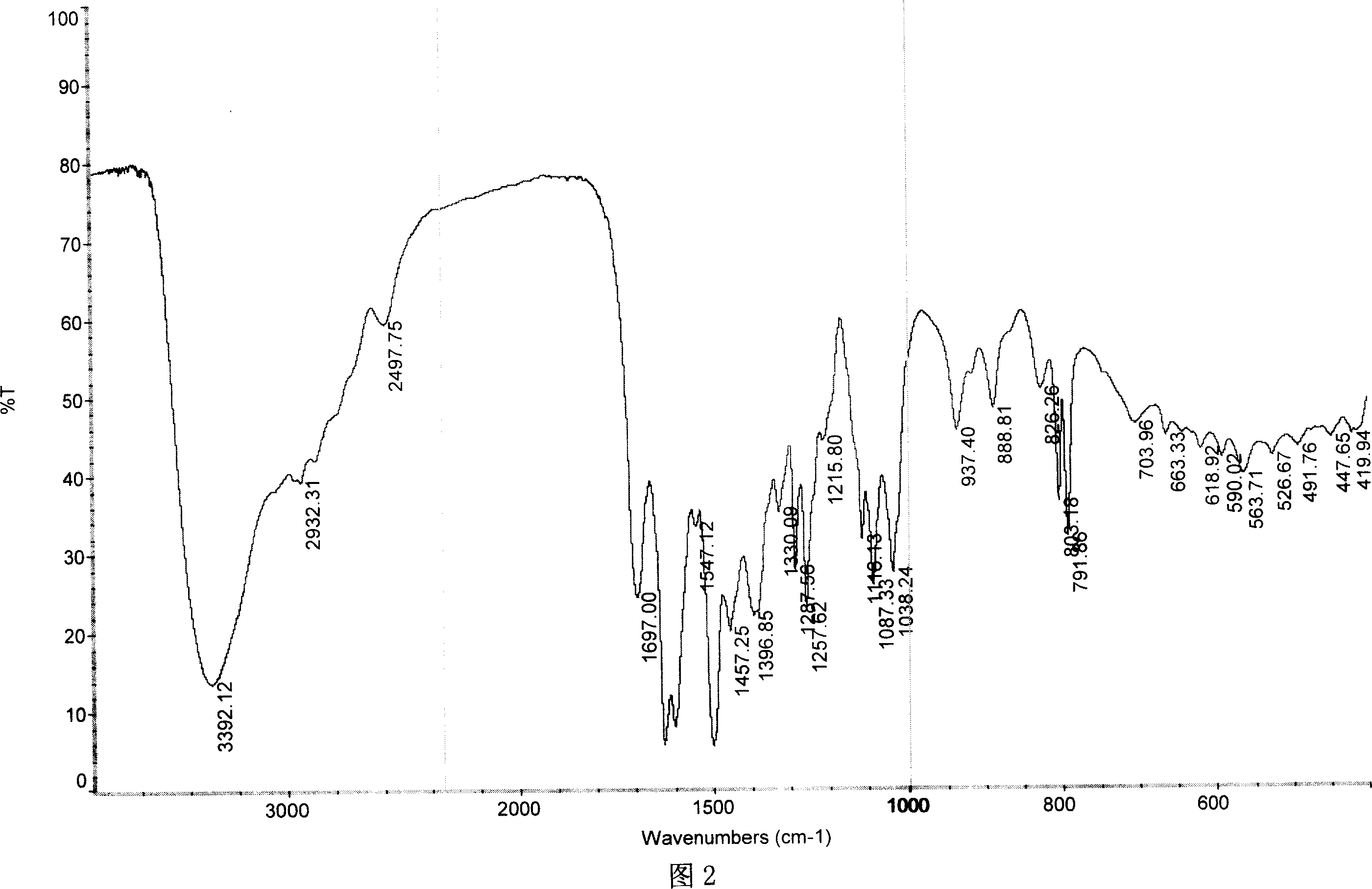
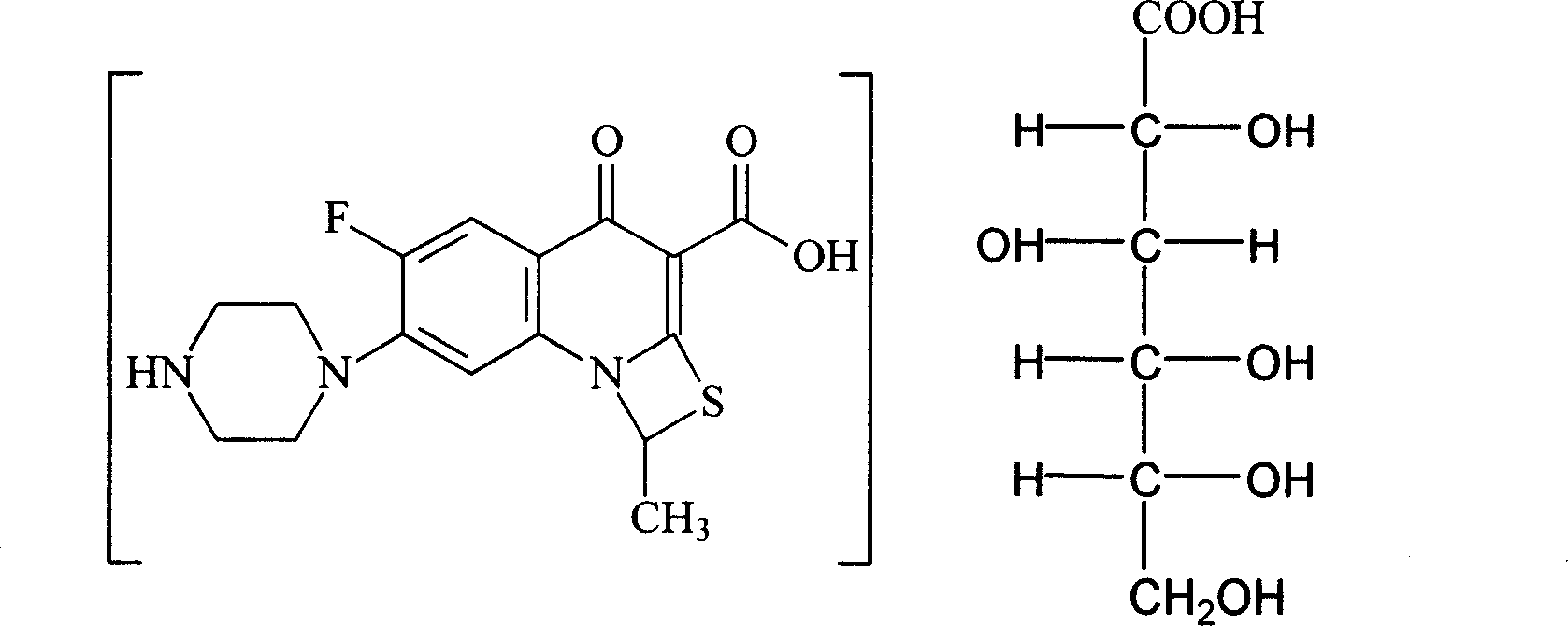
Antibacterial compound in broad spectrum and usage
A technology for compounds and antibacterial drugs, applied in the directions of antibacterial drugs, organic chemistry, drug delivery, etc., can solve problems such as affecting the use of drugs, and achieve the effects of simple production process design, new varieties, and low production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] 15~20°C, add 50L water and 5L isopropanol into a 100L reaction tank, then add 10kg of compound II, add 13L of 50% gluconic acid aqueous solution dropwise under stirring, stir for 30 minutes, add 0.5g of activated carbon for needles, stir for 15 Minutes, filtered into the 500L crystallization tank; 10L water washing reaction tank, filtered into the 500L crystallization tank. Open the crystallization tank and stir, add 400 L of isopropanol dropwise, and finish adding isopropanol in 4 hours, and a solid precipitates out. Keep warm at 15-20°C and stir for 2 hours, filter, wash once with 20L of isopropanol, and vacuum-dry at 35-40°C. 8.9 kg of compound I were obtained.
[0056] The product elemental analysis results are as follows:
[0057] Analysis Project
C
H
N
S
measured value%
48.55
4.86
7.75
5.87
48.67
4.72
7.68
5.79
Calculated%
48.62
...
Embodiment 2
[0061] 10~15°C, add 50L water and 20L isopropanol to the reaction tank, then add 10kg compound II, add dropwise 13.4L of 50% gluconic acid aqueous solution under stirring, stir for 1 hour, add 0.5kg of activated carbon for needles, stir for 60 Minutes, filter and decarburize; the filtrate is pressed into a 1000L crystallization tank in a sterile room through a 0.22μm ultrafiltration membrane. 15L of water washes the reaction tank and pipelines, and is also pressed into the crystallization tank. Stirring was started, and 650 L of acetone was added dropwise, and the addition of acetone was completed in 5 hours, and a solid precipitated. Keep warm at 0-5°C and stir for 5 hours, filter, wash with acetone 30L×2 twice, and vacuum-dry at 35-40°C. Obtain 8.4kg of sterile powder of compound I.
Embodiment 3
[0063] 0~5°C, add 50ml of water to a 100ml three-neck flask, then add 10g of compound II, add 13.2L of 50% gluconic acid aqueous solution dropwise under stirring, stir for 30 minutes, add 0.5g of activated carbon for needles, stir for 15 minutes, filter Decarburization; wash the three-necked flask with 10ml of water, and filter. Combine the filtrates into a 500ml three-neck flask, add 300ml of ethanol dropwise under stirring, and add the mixed solution in 3 hours, and a solid precipitates out. Keep warm at 15-20°C and stir for 2 hours, filter, wash once with 20ml of absolute ethanol, and vacuum-dry at 35-40°C. 6.9 g of compound I are obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com