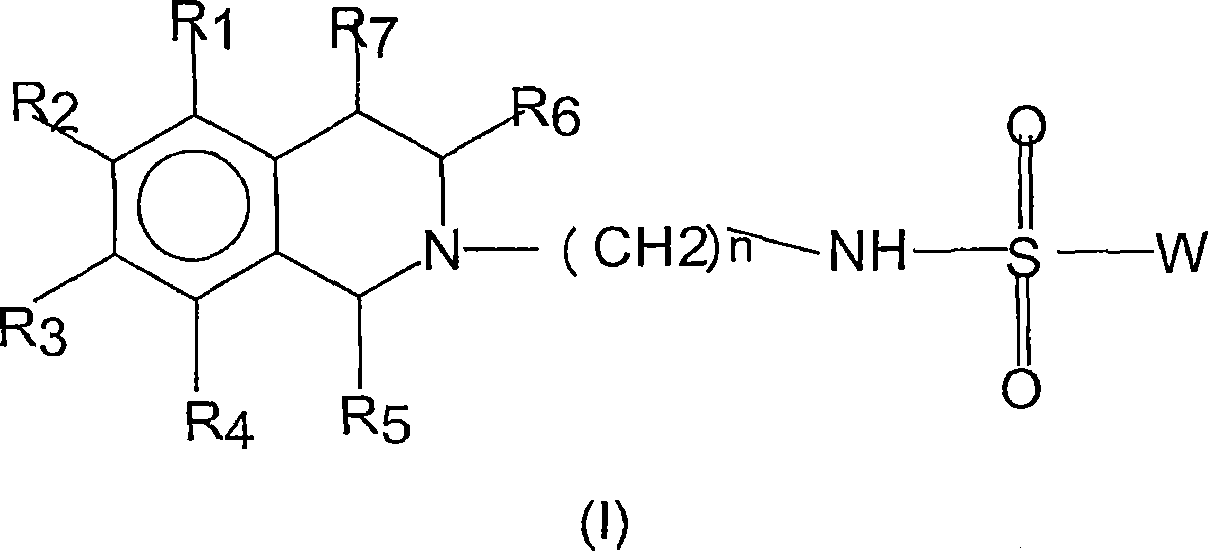
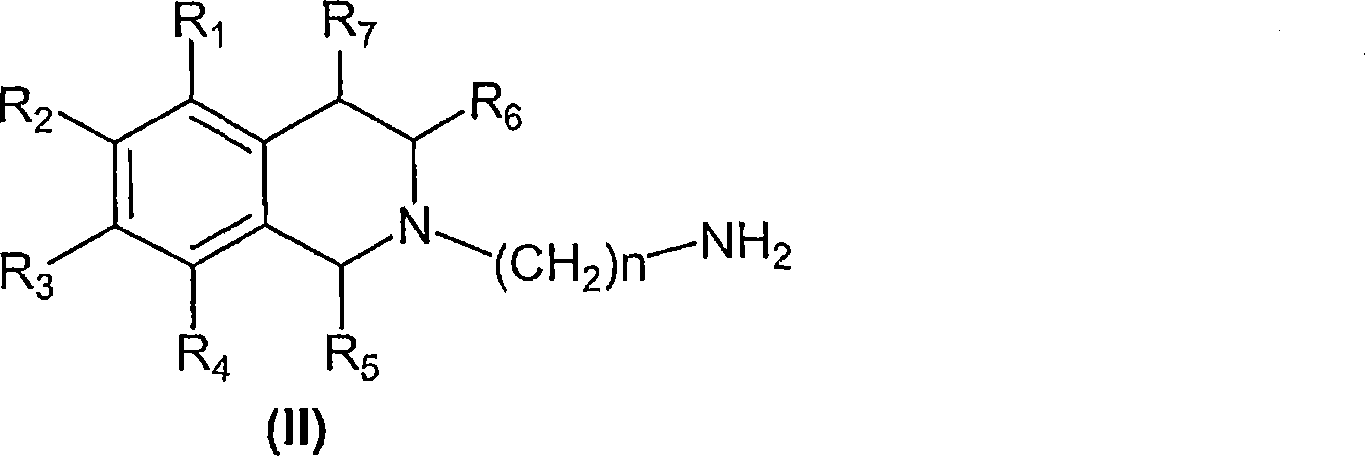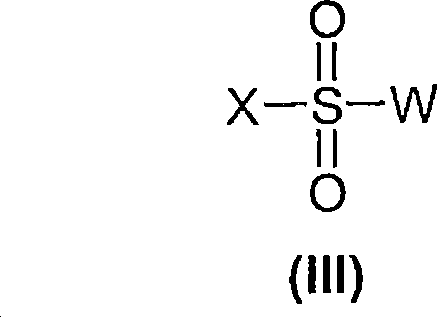5-HT7 receptor antagonosts
A technology of isomers and solvates, applied in the field of 5-HT7 receptor antagonists, can solve problems such as low sequence homology and unreported activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0123] The starting materials of general formula (IV) are prepared by conventional methods of organic chemistry well known to those skilled in the art.
[0124] The synthesis of general formula (IV) compound
[0125] 7-Nitro-1,2,3,4-tetrahydroisoquinoline hydrochloride
[0126] This compound is described in J. Med. Chem. 2003, 46, 831-837 by Buolamwini et al., which is hereby incorporated by reference and forms part of the present disclosure.
[0127]
[0128] A cold solution of 1,2,3,4-tetrahydroisoquinoline (13.3 g, 0.1 mol) in concentrated sulfuric acid (50 mL) was treated in small portions with potassium nitrate (11.12 g, 1.1 mol) maintaining the temperature at 5 below ℃. The reaction was left overnight at room temperature and poured onto ice. The resulting solution was basified with ammonium hydroxide and washed with CH 2 Cl 2 Extracted, dried and evaporated to dryness in vacuo. The crude product was dissolved in 100 mL of ethanol. Then 2.8M hydrochloric acid in...
Embodiment B
[0133] Synthesis of compounds of general formula (II)
[0134]
[0135] a) 2-(4-N-phthalimidobutyl)-7-nitro-1,2,3,4-tetrahydroisoquinoline
[0136] At room temperature, 7-nitro-1,2,3,4-tetrahydroisoquinoline hydrochloride (9.87 g, 0.046 mol), N- A mixture of (4-bromobutyl)phthalimide (12.97 g, 0.046 mol), potassium carbonate (25.43 g, 0.184 mol) was stirred overnight. The mixture was concentrated in vacuo and the residue was dissolved in water (150 mL), extracted with ethyl acetate (3 x 50 mL), washed with water, the organic layer was dried and evaporated to give the product (16.58 g, 95% yield), This product was used without further purification.
[0137] 1 H NMR (300MHz, DMSO-D6) d ppm 1.64(m, 2H), 1.79(m, 2H), 2.60(m, 2H), 2.78(m, 2H), 2.96(m, 2H), 3.72(m, 4H), 7.23 (m, 1H), 7.71 (m, 2H), 7.79 (m, 3H), 8.01 (m, 1H).
[0138] b) 2-(4-aminobutyl)-7-nitro-1,2,3,4-tetrahydroisoquinoline dihydrochloride
[0139]2-(4-N-phthalimidobutyl)-7-nitro-1,2,3,4-tetrahydroisoquino...
Embodiment C
[0144] Compounds of general formula (I) are prepared by coupling compounds of general formula (II) with compounds of general formula (III) according to conventional methods of organic chemistry known to those skilled in the art.
[0145] Naphthalene-1-sulfonic acid [4-(3,4-dihydro-1H-isoquinolin-2-yl)-butyl]amide hydrochloride
[0146]
[0147] Naphthalene-1-sulfonyl chloride (238 mg, 1.05 mmol) was added to 2-(4-aminobutyl)-1,2,3,4-tetrahydroisoquinoline dihydrochloride (272 mg, 1 mmol), N, CH of N-diisopropylethylamine (517mg, 4mmol) 2 Cl 2 (10 mL) solution and stirred overnight at room temperature. The resulting solution was washed with water (3×15 mL), washed with Na 2 SO 4 The organic layer was dried and evaporated to dryness. The free base was dissolved in 2-propanol (5 mL). Then 2.8 M ethanolic hydrogen chloride solution (0.40 mL) was added. The product crystallized and collected by filtration and dried in vacuo to give a white solid (310 mg, 72%).
[0148] M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com