Biological activity of pigment epithelium-derived factor and methods of use
A homologous, angiogenesis technology, applied in the analysis of biological materials, medical preparations containing active ingredients, drug combinations, etc., can solve the problem of PEDF lacking protease inhibitory activity and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0126] Formulations of the pharmaceutical compositions described herein may be prepared by any of the methods known or later developed in the art of medicine. In general, such preparation methods include the steps of bringing into association the active ingredient with the carrier or one or more other additional ingredients, and then, if necessary or more appropriate, shaping or packaging the product into desired single or multiple forms. dosage unit.
[0127] Although the description of the pharmaceutical compositions provided herein is primarily directed to pharmaceutical compositions suitable for administration to humans by prescription, those skilled in the art will appreciate that such compositions are generally suitable for administration to all species of animals . Known and generally skilled veterinary pharmacologists, if necessary, can only use any ordinary experiments to design and modify the pharmaceutical composition suitable for administration to humans in order ...
Embodiment 1
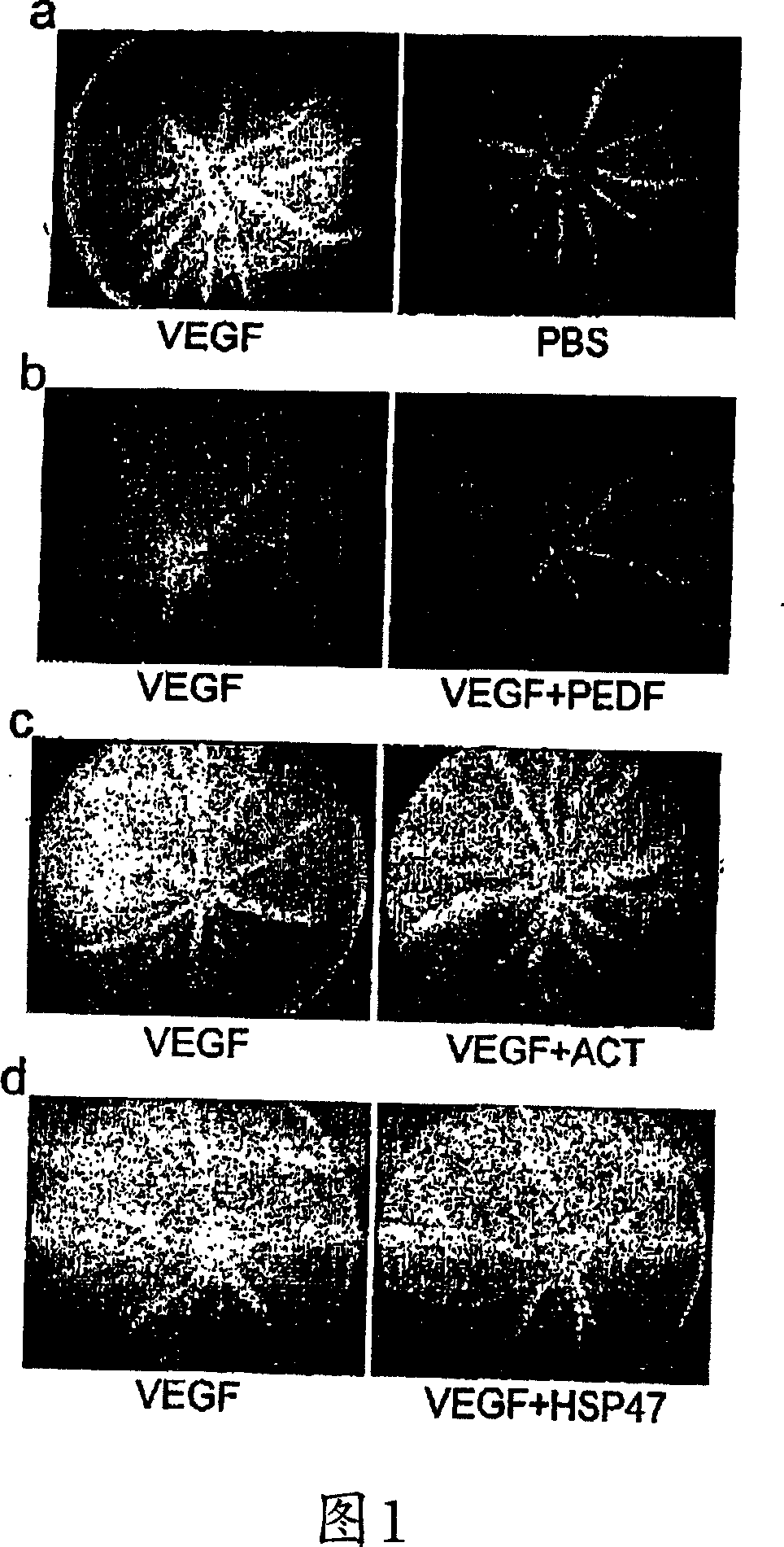
[0177] Example 1: PEDF qualitatively inhibits VEGF-induced retinal vascular permeability
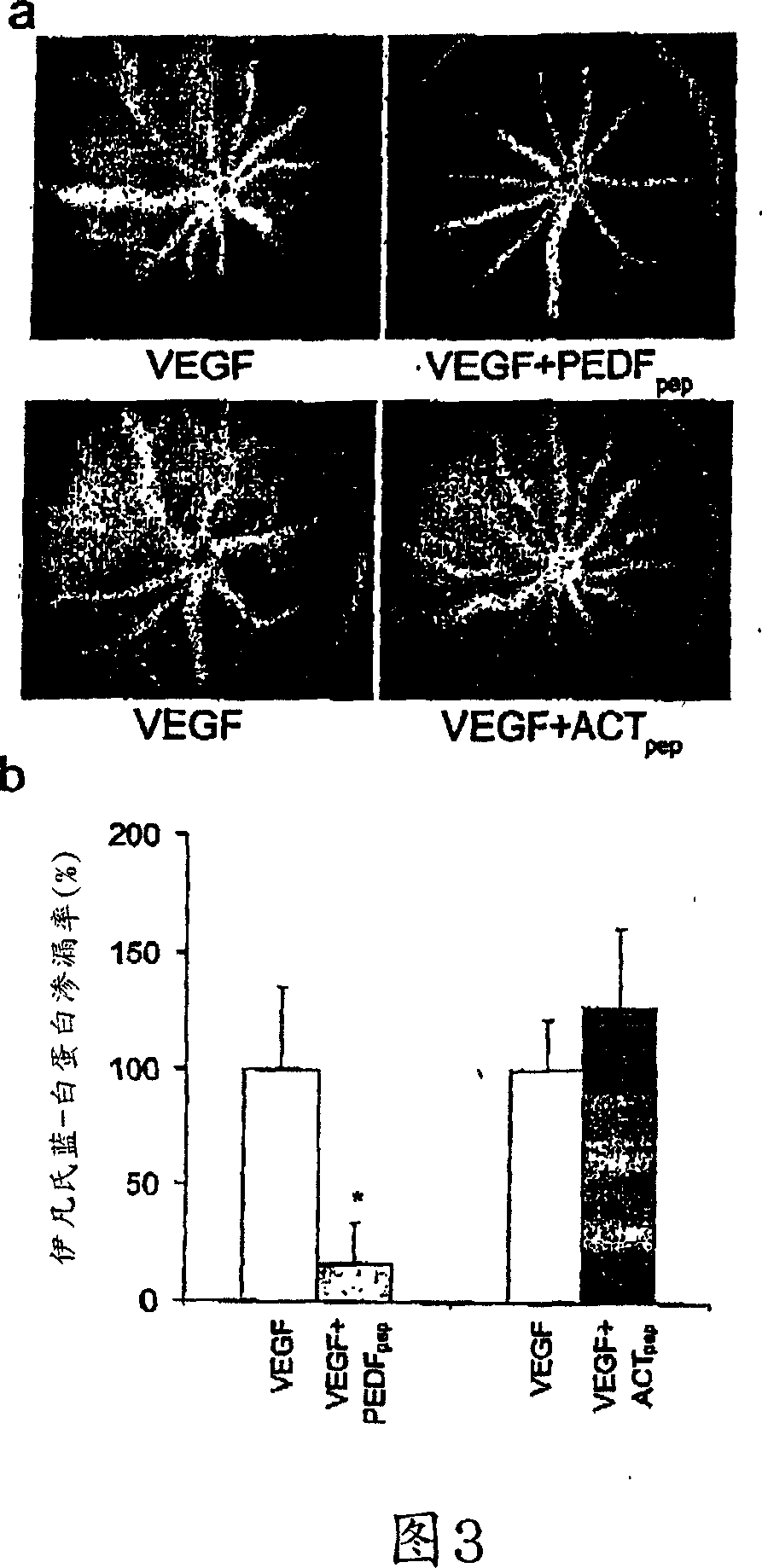
[0178] Luciferin angiography is a clinical diagnostic technique that allows us to visualize the effects of factors that regulate VEGF-induced permeability. Decreased fluorescence in one eye relative to the other eye on the contralateral side may be attributable to agents injected into both eyes. Vascular permeability due to VEGF 28 , so as expected, compared with the fellow eye injected with saline, receiving VEGF 164 (human VEGF in mouse 165 Eyes with an ortholog) showed increased luciferin leakage (Fig. 1a). When PEDF and VEGF 164 When co-injected, no VEGF-induced vascular permeability was observed (Fig. 1b).
[0179] To show that the antivascular permeability activity is specific for PEDF, we tested the effect of ACT and HSP47 in the same assay. ACT and HSP47 are two subfamilies from the serine protease inhibitor (serpin) superfamily 29 , which is different from the subfamily...
Embodiment 2
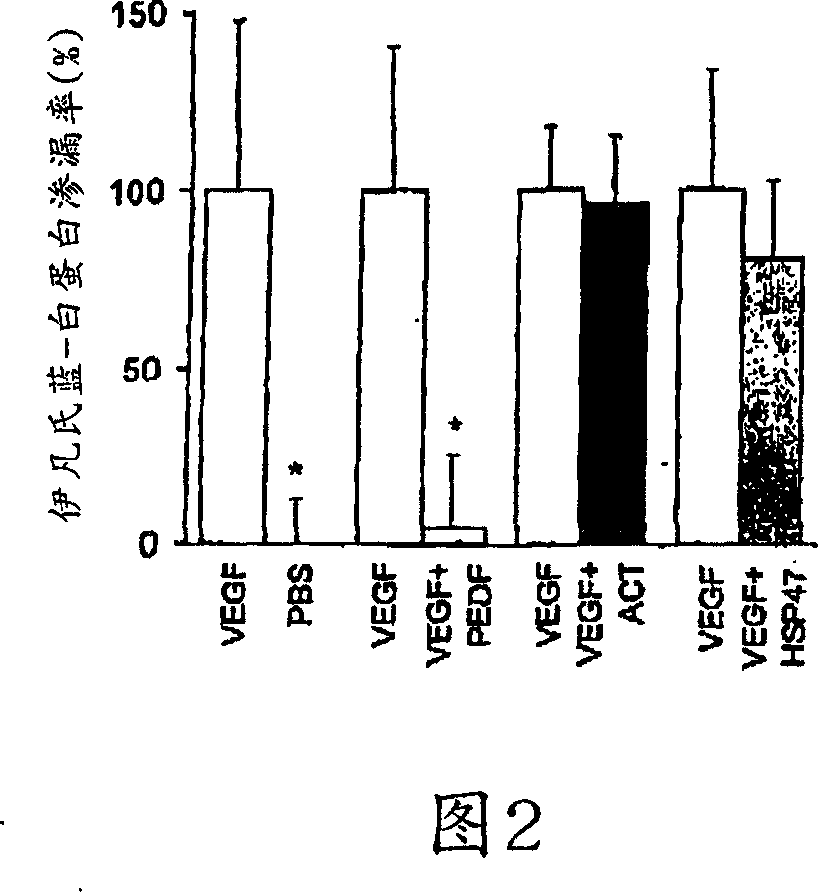
[0180] Example 2: PEDF Quantitatively Inhibits VEGF-Induced Retinal Vascular Permeability
[0181] To quantify and confirm the ability of PEDF to inhibit VEGF-induced vascular permeability, we used a modified Evans blue assay 32 . Mice injected intravitreally as in luciferin angiography experiments received Evans blue intravascularly 24 hours later. PEDF almost terminated (95.6±21.2%) VEGF-induced vascular permeability, while ACT and HSP47 had no discernible effect (3.4±18.2% and 19.4±22.3% inhibition, respectively) ( FIG. 2 ). These data quantitatively confirm what we observed qualitatively by luciferin angiography: PEDF inhibits VEGF-induced vascular permeability.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com