Methods for treating conditions associated with MASP-2 dependent complement activation
A technology of MASP-2 and complement activation, which is applied in chemical instruments and methods, biochemical equipment and methods, and resistance to vector-borne diseases, etc. It can solve the complexity and diversity of carbohydrate structures and it is difficult to determine the shared molecular determinants And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0436] This example describes the generation of a mouse strain deficient in MASP-2 (MASP-2- / -) but sufficient in MAp19 (MAp19+ / +).
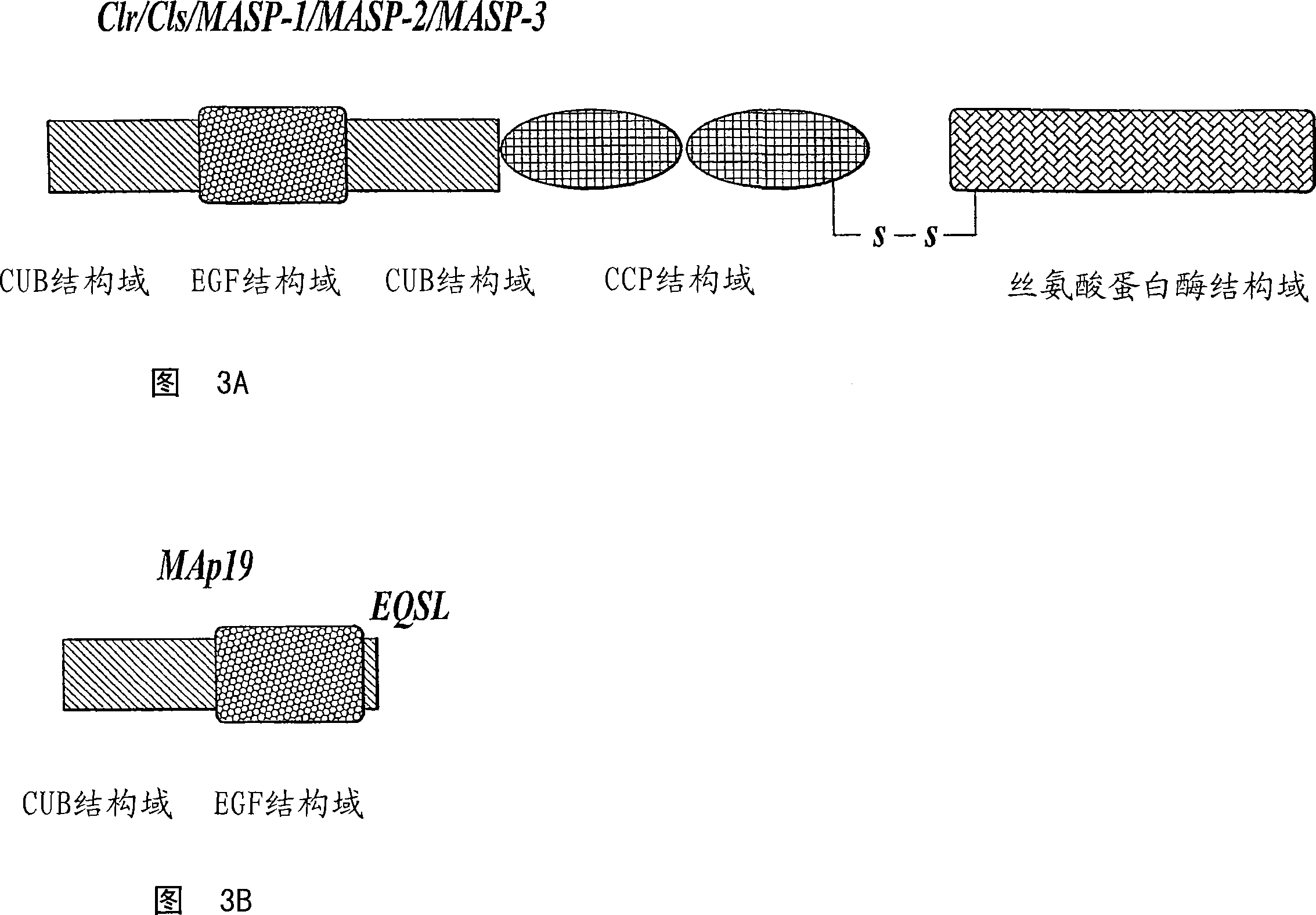
[0437] Materials and methods: The targeting vector pKO-NTKV 1901 was designed to disrupt the three exons encoding the C-terminus of murine MASP-2, including the exon encoding the serine protease domain, as shown in Figure 4. PKO-NTKV 1901 was used to transfect the murine ES cell line E14.1a (SV129Ola). Neomycin resistant and thymidine kinase sensitive clones were selected. 600 ES clones were screened, among which four different clones were identified, which were confirmed by southern blotting to contain the expected selective targeting and recombination results, as shown in FIG. 4 . Chimeras were generated from these four positive clones by embryo transfer. The chimeras were then backcrossed in the genetic background C57 / BL6 to generate transgenic males. Transgenic males were crossed with females to produce F1, and 50% of the offspring showed...
Embodiment 2
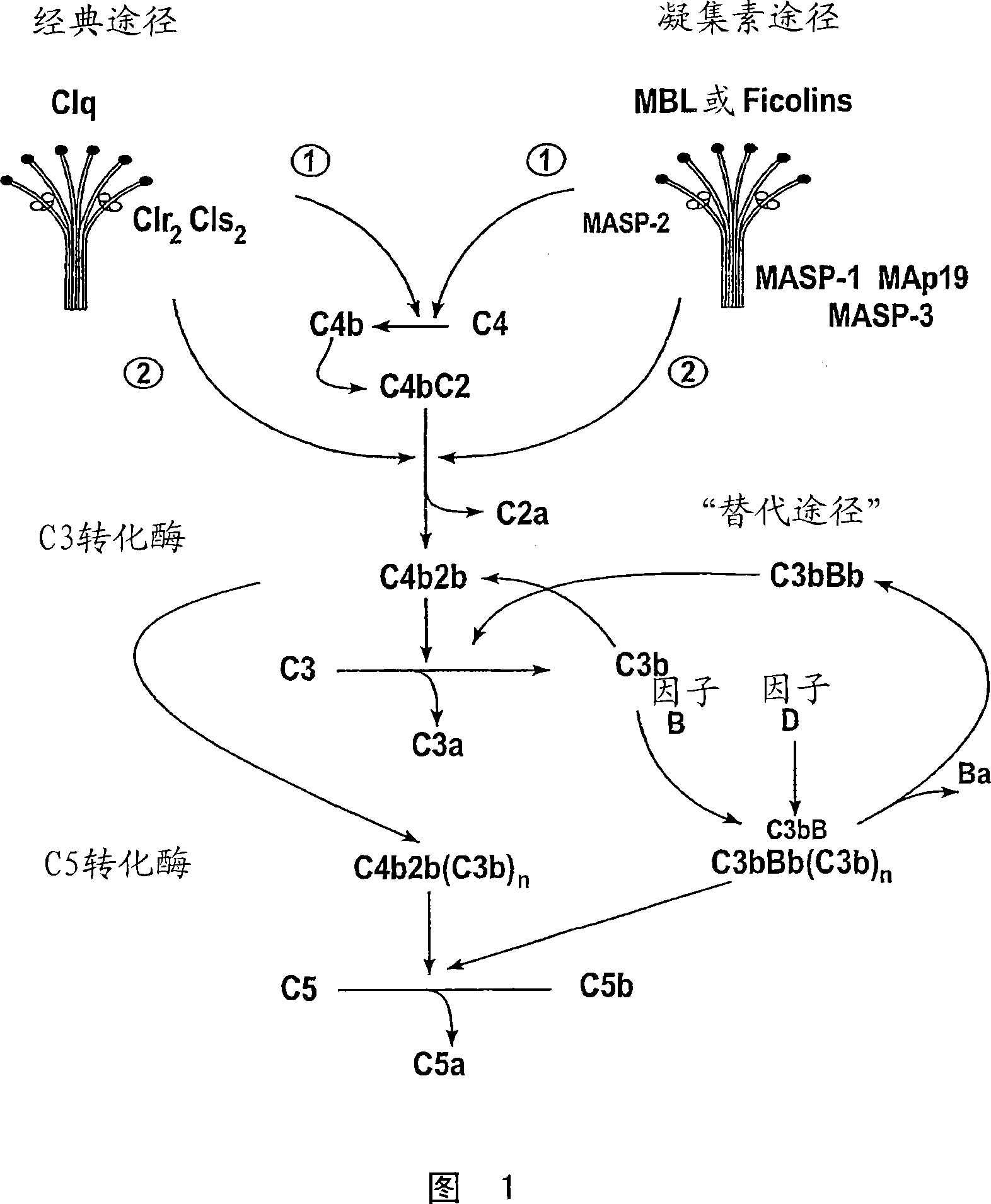
[0441] This example demonstrates that MASP-2 is required for complement activation via the alternative and lectin pathways.
[0442] Methods and materials:
[0443] Lectin pathway-specific C4 cleavage assays: The C4 cleavage assay has been described by Petersen, et al., J. Immunol. Methods 257:107 (2001), which measures the production of L-ficolin-bound lipoteichoic acid (LTA) from Staphylococcus aureus. Lectin pathway activation. The assay described in Example 11 was modified to measure lectin pathway activation by MBL by coating plates with LPS and mannan or zymosan, followed by addition of serum from MASP-2- / - mice, as described below. The assay was also modified to eliminate the possibility of C4 cleavage due to the classical pathway. This can be achieved by using a sample dilution buffer containing 1M NaCl, which allows high-affinity binding of the lectin pathway recognition components to their ligands, but prevents activation of endogenous C4, thereby dissociating...
Embodiment 3
[0470] This example describes the generation of a transgenic mouse line that is murine MASP-2- / -, MAp19+ / +, and expresses a human MASP-2 transgene (mouse MASP-2 knockout, human MASP-2 knockin).
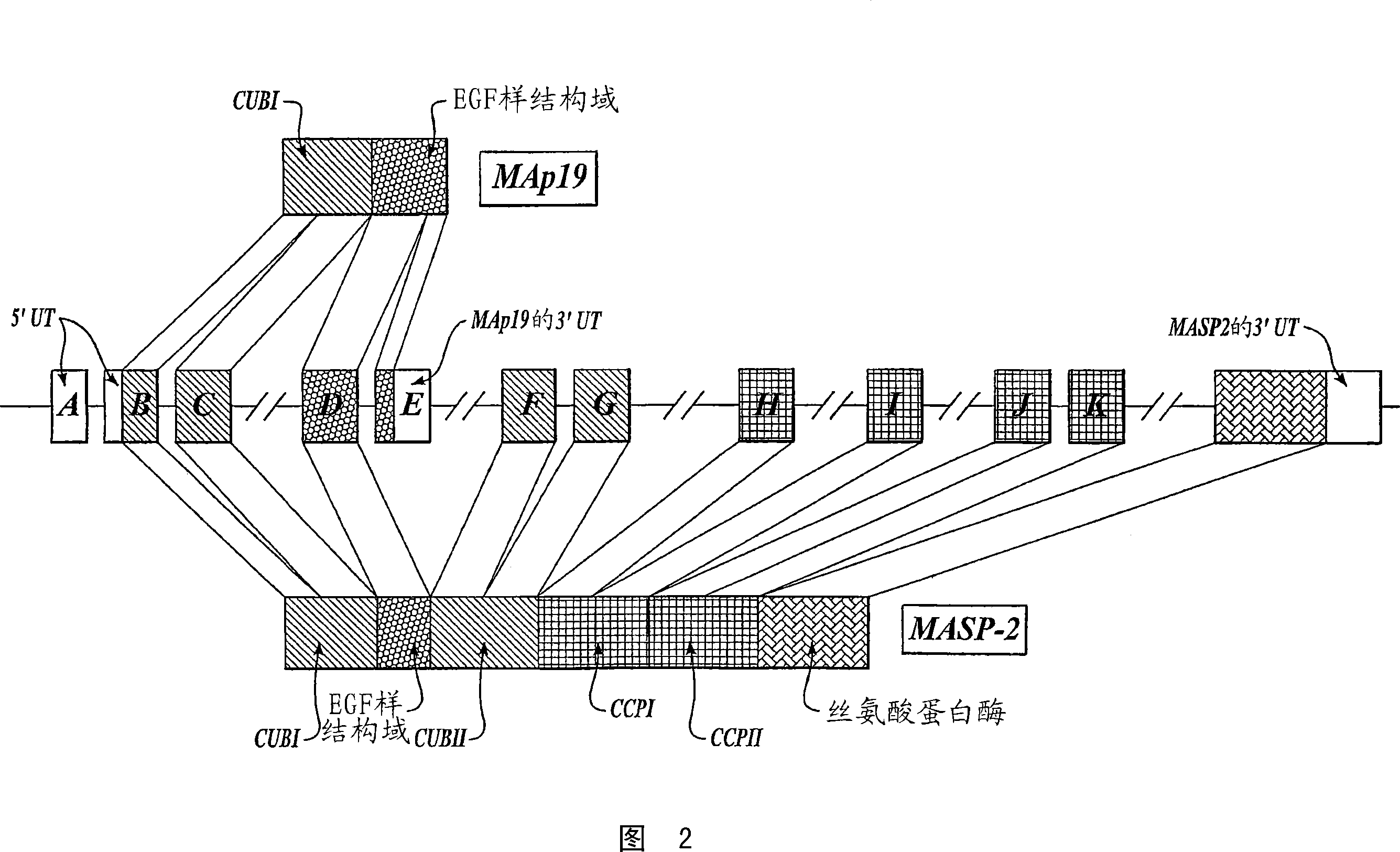
[0471] Materials and methods: A miniature gene (SEQ ID NO: 49) encoding human MASP-2 called "miniature hMASP-2" shown in Figure 5 was constructed, which included the promoter region of the human MASP 2 gene, including the first 3 exons ( Exon 1 to Exon 3) is followed by a cDNA sequence representing the coding sequence for the next 8 exons, encoding the full-length MASP-2 protein driven by its endogenous promoter. The miniature hMASP-2 construct was inserted into MASP-2- / - zygotes to transgenically express human MASP-2 in place of the missing murine MASP 2 gene.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com