Medicine composition of alprostadil and Chuanhuning/Yanhuning liposome and its prepn
A technology of alprostadil and Yanhuning, applied in the field of alprostadil and Chuanhuning/Yanhuning liposome combination medicine and preparation thereof, can solve the problems of pyrogen-like reaction, allergic reaction, liver function damage, etc. Use dose, improve the therapeutic index, and promote the effect of hepatocyte regeneration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] This embodiment provides a kind of nano liposome drug, comprising alprostadil and Chuanhuning / Yanhuning, comprising the following raw materials in parts by weight:
[0031] Alprostadil and Chuanhuning / Yanhuning
[0032] Mixture 80.2 in a weight ratio of 0.1:300;
[0033] 4:1 mixture of soybean lecithin and egg yolk lecithin in weight ratio 440;
[0034] Cholesterol and β-sitosterol weight ratio 9: 1 mixture 200;
[0035] Macrogol 4000 40;
[0036] Sodium Glucuronate 280;
[0037] Vitamin C 800.
[0038] The Chuanhuning / Yanhuning refers to the mixture of Chuanhuning and Yanhuning in a weight ratio of 3:1 or Chuanhuning alone.
[0039] The preparation method of above-mentioned medicine, the steps are as follows:
[0040] (1) Get the mixture of Alprostadil and Chuanhuning / Yanhuning weight ratio 0.1: 300, soybean lecithin and egg yolk lecithin mixture, cholesterol and β-sitosterol weight ratio 9: 1 mixture dissolved in ethanol or ether In, make a solution;
[0041] ...
Embodiment 2
[0052] This embodiment provides a kind of nano liposome drug, comprising alprostadil and Chuanhuning / Yanhuning, comprising the following raw materials in parts by weight:
[0053] Alprostadil and Chuanhuning / Yanhuning
[0054] Mixture 10.1 in a weight ratio of 0.1:500;
[0055] 4:1 mixture of soybean lecithin and egg yolk lecithin by weight 88;
[0056] 9:1 mixture of cholesterol and β-sitosterol by weight 40;
[0057] Polyethylene glycol 4000 15;
[0058] Sodium Glucuronate 100;
[0059] Vitamin C 160.
[0060] The Chuanhuning / Yanhuning refers to the mixture of Chuanhuning and Yanhuning in a weight ratio of 2:1 or Chuanhuning alone.
[0061] The preparation method of above-mentioned medicine refers to embodiment 1.
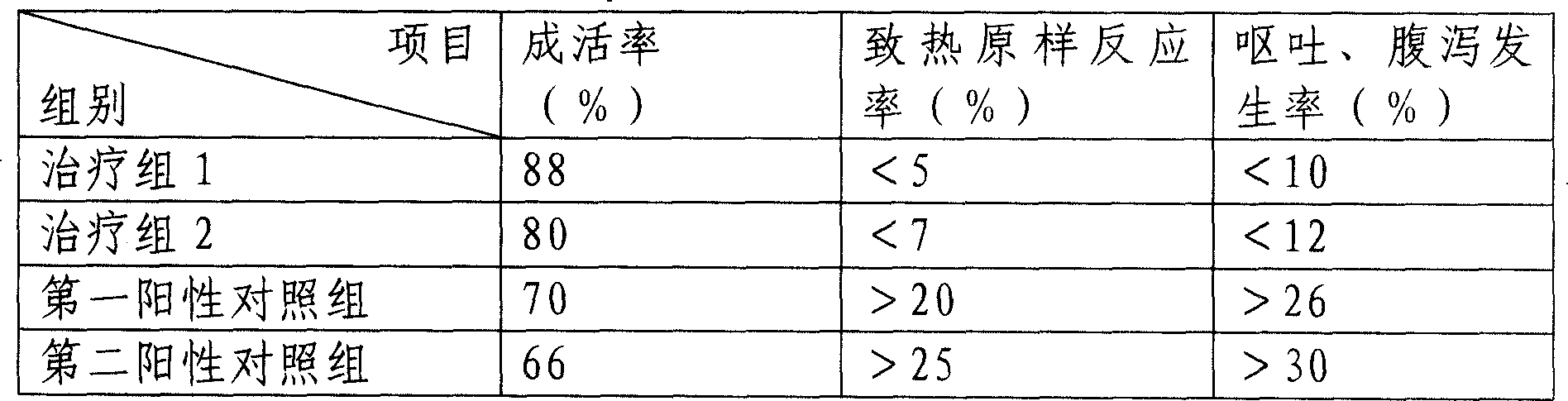
[0062] The curative effect verification of the medicine prepared in the present embodiment is as follows:
[0063] Induce pneumonia model mice with A1 type influenza virus, 18-20 grams in weight, with 20μg / kg alprostadil / 10mg / kg Yanhuning / Chuanhuning (trea...
Embodiment 3
[0067] This embodiment provides a kind of nano liposome drug, comprising alprostadil and Chuanhuning / Yanhuning, comprising the following raw materials in parts by weight:
[0068] Alprostadil and Chuanhuning / Yanhuning
[0069] Mixture 80.2 in weight ratio 1.0:266;
[0070] 4:1 mixture of soybean lecithin and egg yolk lecithin by weight 88;
[0071] Cholesterol and β-sitosterol weight ratio 9: 1 mixture 200;
[0072] Polyethylene glycol 4000 15;
[0073] Sodium Glucuronate 100;
[0074] Vitamin C 800.
[0075] The Chuanhuning / Yanhuning refers to the mixture of Chuanhuning and Yanhuning in a weight ratio of 3:1 or Yanhuning alone.
[0076] The preparation method of above-mentioned medicine refers to embodiment 1.
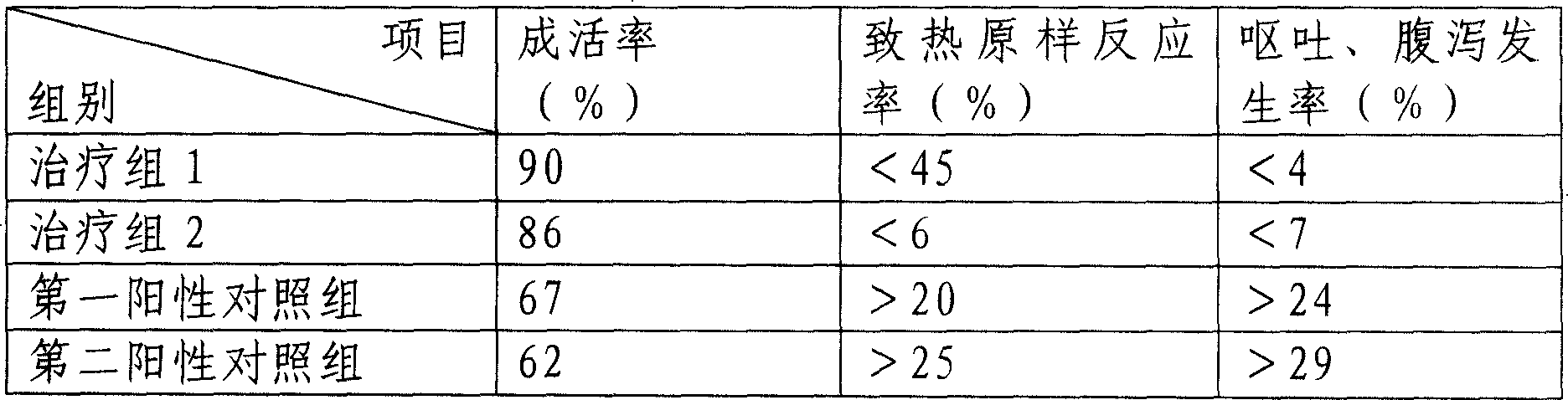
[0077] The curative effect verification of the medicine prepared in the present embodiment is as follows:
[0078] Induce pneumonia model mice with A1 type influenza virus, 18-20 grams in weight, with 300μg / kg alprostadil 80mg / kg Yanhuning / Chuanhuning (treatmen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
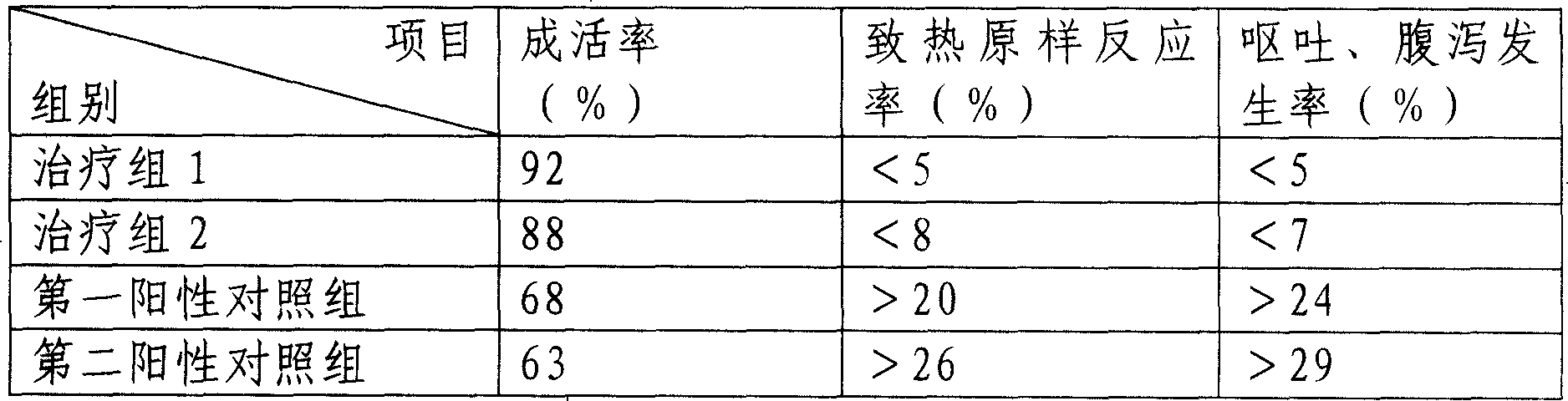
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com