Technology for preparing medicine and relative oral preparations
A technology for preparation process and related products, which is applied in the field of preparation process for oral preparations, can solve problems such as difficult acceptance by patients, achieve the effects of accelerating dissolution and absorption, and improving bioavailability and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
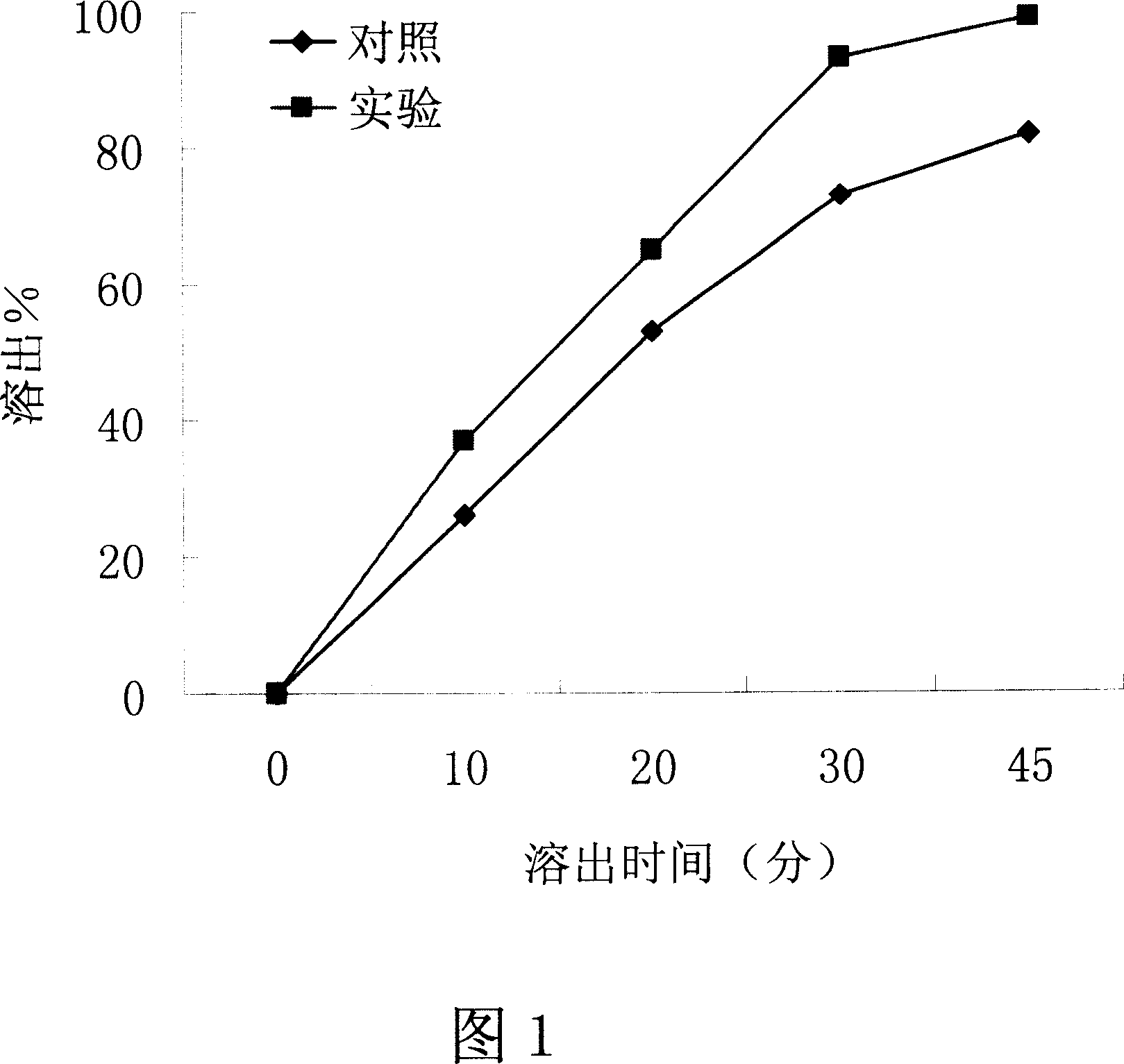
[0038] Embodiment 1-influence of the present invention on the dissolution property of water-insoluble drug
[0039] Drug: Irbesartan, a water-insoluble drug.
[0040] Dosage Form: Tablet
[0041] method:
[0042] 1. The preparation process of the control group: take 15 grams of irbesartan, 5 grams of pregelatinized starch, 6 grams of micropowder silica gel, 26 grams of polyethylene glycol 6000, mix well, wet granulate with 10% PVPk30, and dry at 37 ° C , through a 20-mesh sieve, add 9 grams of microcrystalline cellulose, 8.5 grams of sodium carboxymethyl starch, and 0.5 grams of magnesium stearate, fully stir and mix evenly, and press 0.35g irbesartan tablets on a tablet machine, each tablet Contains irbesartan 75mg.
[0043] 2. The preparation process of the experimental group of the present invention: respectively take 15 grams of irbesartan, 5 grams of pregelatinized starch, 6 grams of micropowdered silica gel, and 26 grams of polyethylene glycol 6000, mix them evenly, a...
Embodiment 2
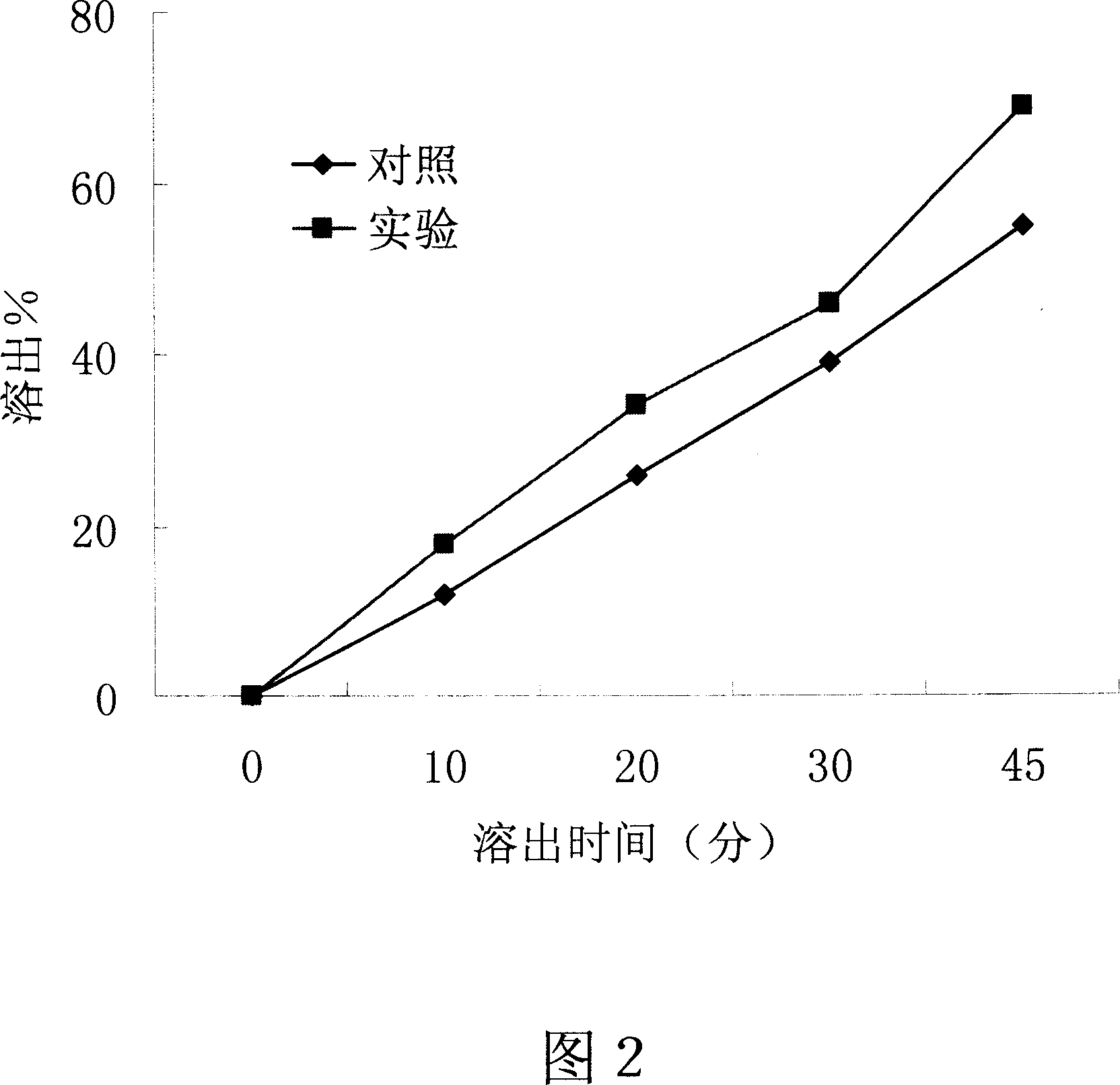
[0046] Embodiment 2-the influence of the present invention on the dissolution of poorly soluble drugs
[0047] Drug: propafenone hydrochloride is a poorly water-soluble drug.
[0048] Dosage Form: Tablet
[0049] method:
[0050] 1. The preparation process of the control group: take 15 grams of propafenone hydrochloride, 3 grams of polyethylene glycol 4000, and 12 grams of polyethylene glycol 6000 respectively, mix well, dry granulate, pass through a 20-mesh sieve, and press heavy 0.3g propafenone hydrochloride tablets, each containing 150 mg propafenone hydrochloride.
[0051] 2. The preparation process of the experimental group of the present invention: take 15 grams of propafenone hydrochloride, 3 grams of polyethylene glycol 4000, and 12 grams of polyethylene glycol 6000, mix well, and heat the prepared raw and auxiliary material mixture by electric heating To a molten state, cooled and solidified, granulated, passed through a 20-mesh sieve, and pressed on a tablet mach...
Embodiment 3
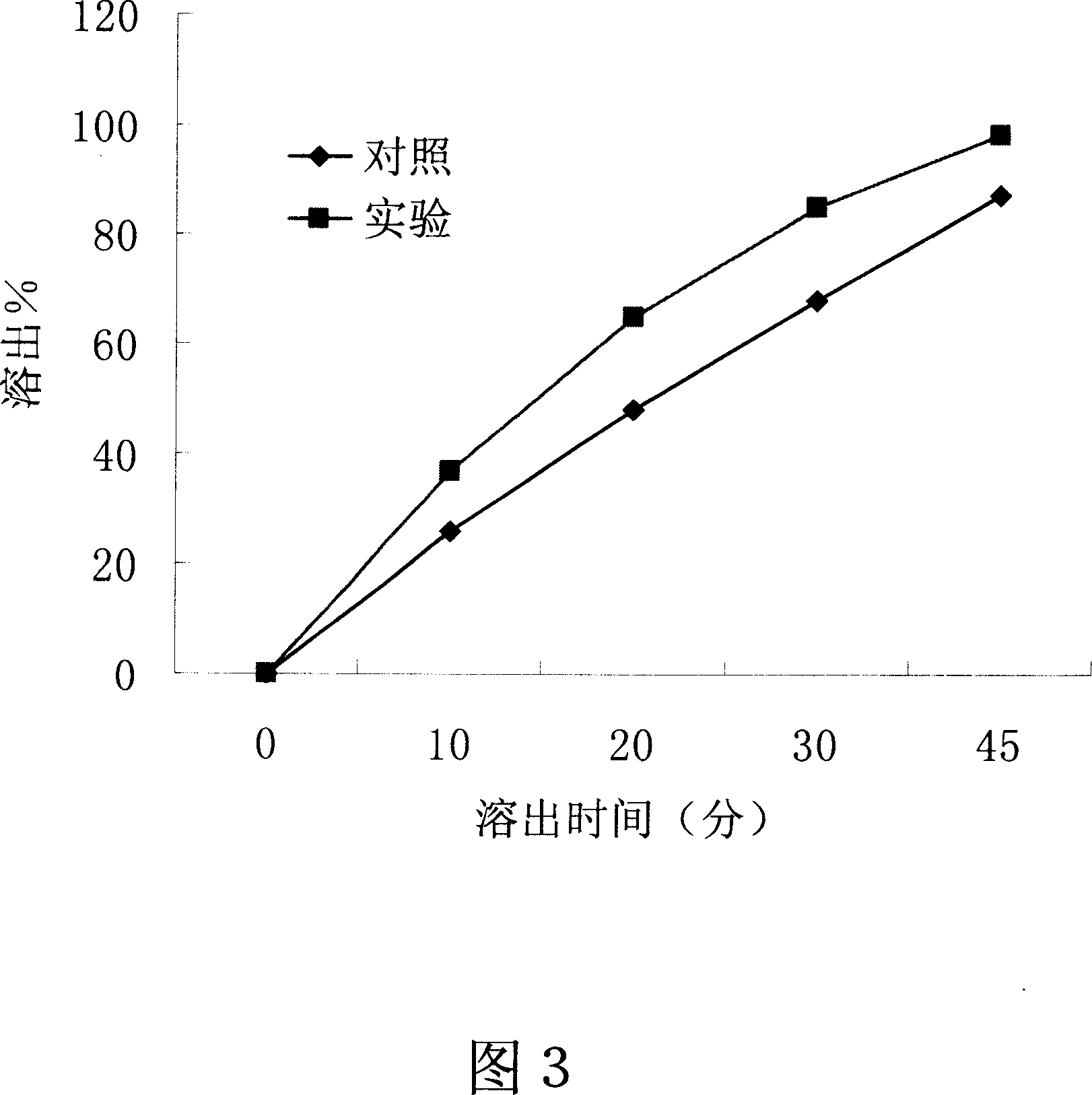
[0054] Embodiment 3-the influence of the present invention on the dissolution of slightly soluble drugs in water
[0055] Drug: Amoxicillin, a slightly soluble drug in water.
[0056] Dosage Form: Tablet
[0057] method:
[0058] 1. The preparation process of the control group: respectively take 15 grams of amoxicillin, 3 grams of polyethylene glycol 4000, and 12 grams of polyethylene glycol 6000, mix well, dry granulate, pass through a 20-mesh sieve, and press on a tablet machine with a weight of 0.3 g Amoxicillin tablets, each containing 150 mg of amoxicillin.
[0059] 2, the preparation process of the experimental group of the present invention: take respectively 15 grams of amoxicillin, 3 grams of polyethylene glycol 4000, and 12 grams of polyethylene glycol 6000, and mix them evenly; Molten state, cooled and solidified, granulated, passed through a 20-mesh sieve, and pressed on a tablet machine to weigh 0.3g amoxicillin tablets, each containing 150 mg of amoxicillin. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com