Artificial antigen submit cell and preparation method thereof
An artificial antigen and cell technology, applied to cells modified by introducing foreign genetic material, using vectors to introduce foreign genetic material, recombinant DNA technology, etc., can solve the problems of increasing treatment costs and achieve the goal of prolonging survival and strengthening interactions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
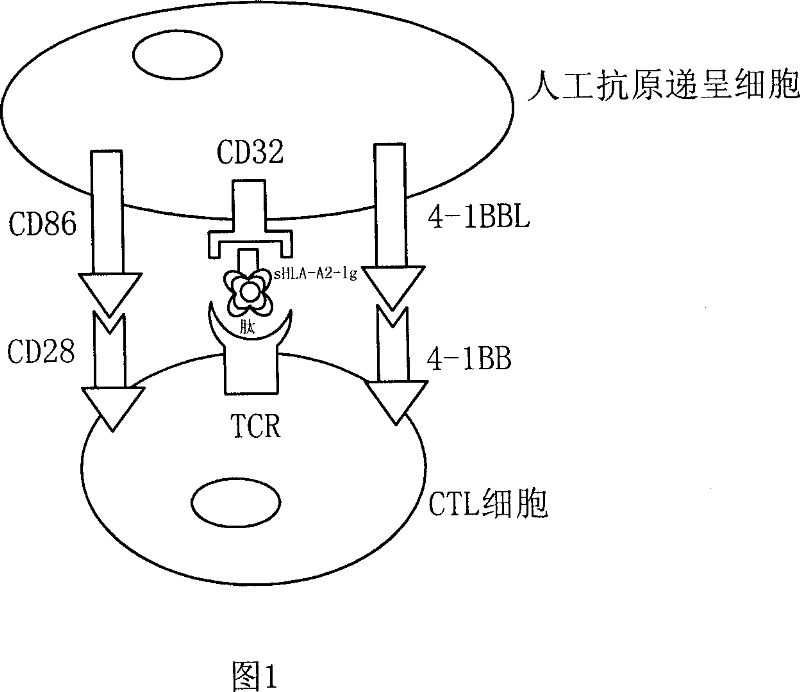
[0045] 1. Construct the cDNA expression vector of CD32, transfect the CD32 recombinant plasmid into K562 cells, screen with G418, identify and sort by FACS, and obtain K32 cells.
[0046] 1. Acquisition of CD32a cDNA: U937 cells in logarithmic phase were taken, RNA was routinely extracted with trizol, and mRNA was reverse transcribed to generate cDNA with Oligod(T)n as a primer. Primers for amplifying CD32a were designed with primer5.0 software, and HindIII and EcoRI restriction sites and protective bases were added to both ends.
[0047] The upstream primer sequence is cgcaagcttg atggctatgg agacccaaat g
[0048] The downstream primer sequence is ccggaattca gcaagctgag agtatgacca c
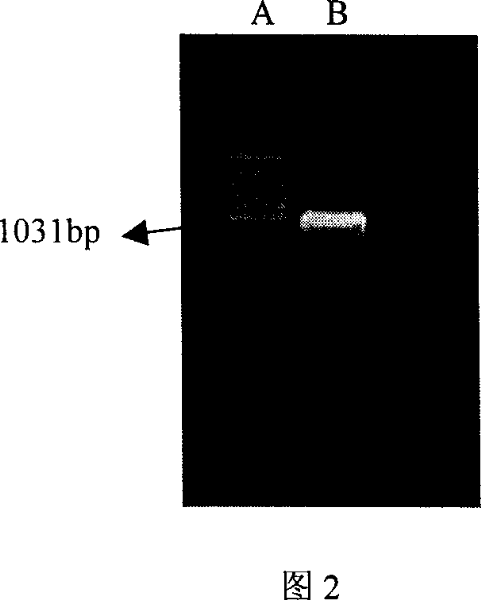
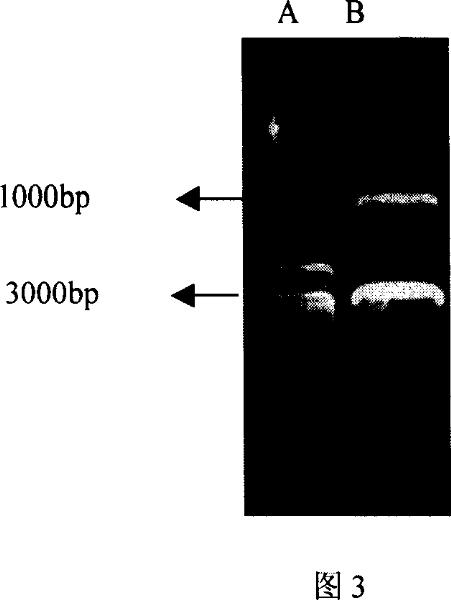
[0049] The PCR amplification program was pre-denaturation at 94°C for 5 minutes, followed by 30 seconds at 94°C, 1 minute at 55°C, and 90 seconds at 72°C for a total of 33 cycles, and a final extension for half an hour. The product of CD32a cDNA was obtained, and the fragment size was about 1000b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com