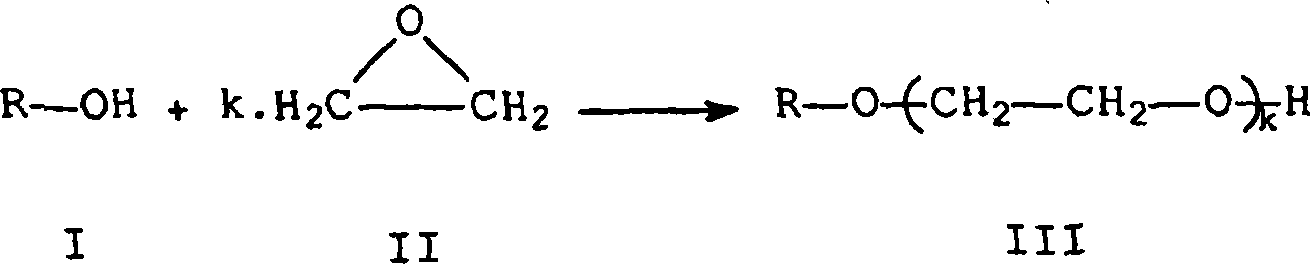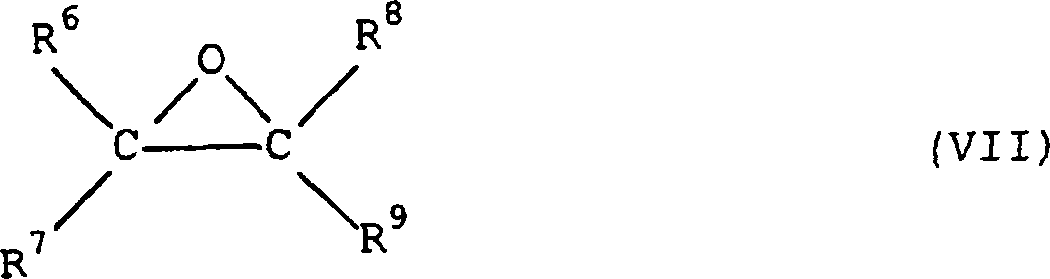Process for preparing an alkoxylated alcohol or phenol
A technology for alkoxylation of alcohols and alkylene oxides, which is applied in the fields of ether preparation, alkylene oxide preparation of ethers, organic chemistry, etc., and can solve the problem of no alkoxylation of secondary or tertiary alcohols
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1 - Ethoxylated secondary alcohol 2-undecanol
[0060]To a "Teflon" bottle equipped with a magnetic stir bar and immersed in a (water) cooling bath was added 2-undecanol (58 mmol, 10 g), boric acid (50 mg) and hydrogen fluoride (50 mg). Ethylene oxide was added in the gas phase at atmospheric pressure at such a flow that the temperature did not exceed 50°C. After about 3 hours, 15.8 g of ethylene oxide (358 mmol) were consumed, which corresponds to a degree of ethoxylation of 6.2 based on the amount introduced, and the product was then treated with about 50 mg of diethanolamine. The yield of ethoxylated 2-undecanol was 0.316 kg EO / g HF.
[0061] Measurements of average moles of ethylene oxide per mole of 2-undecanol, ethoxylate distribution and residual free alcohol were performed using high performance liquid chromatography (HPLC). The technique for these measurements involves the derivatization of ethoxylated alcohols with 4-nitrobenzoyl chloride. The produ...
Embodiment 2
[0063] Ethoxylated secondary alcohol 2-undecanol
[0064] The ethoxylation of 2-undecanol was carried out using the method of Example 1, except that the reaction temperature was maintained at 70°C. Measurements of average moles of ethylene oxide per mole of 2-undecanol, ethoxylate distribution and residual free alcohol were performed using the same technique as used in Example 1. The results are shown in Table 1 below.
Embodiment 3
[0066] Ethoxylated secondary alcohol 2-undecanol
[0067] The ethoxylation of 2-undecanol was carried out using the method of Example 1, except that the reaction temperature was maintained at 130°C. Measurements of average moles of ethylene oxide per mole of 2-undecanol, ethoxylate distribution and residual free alcohol were performed using the same technique as used in Example 1. The results are shown in Table 1 below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com