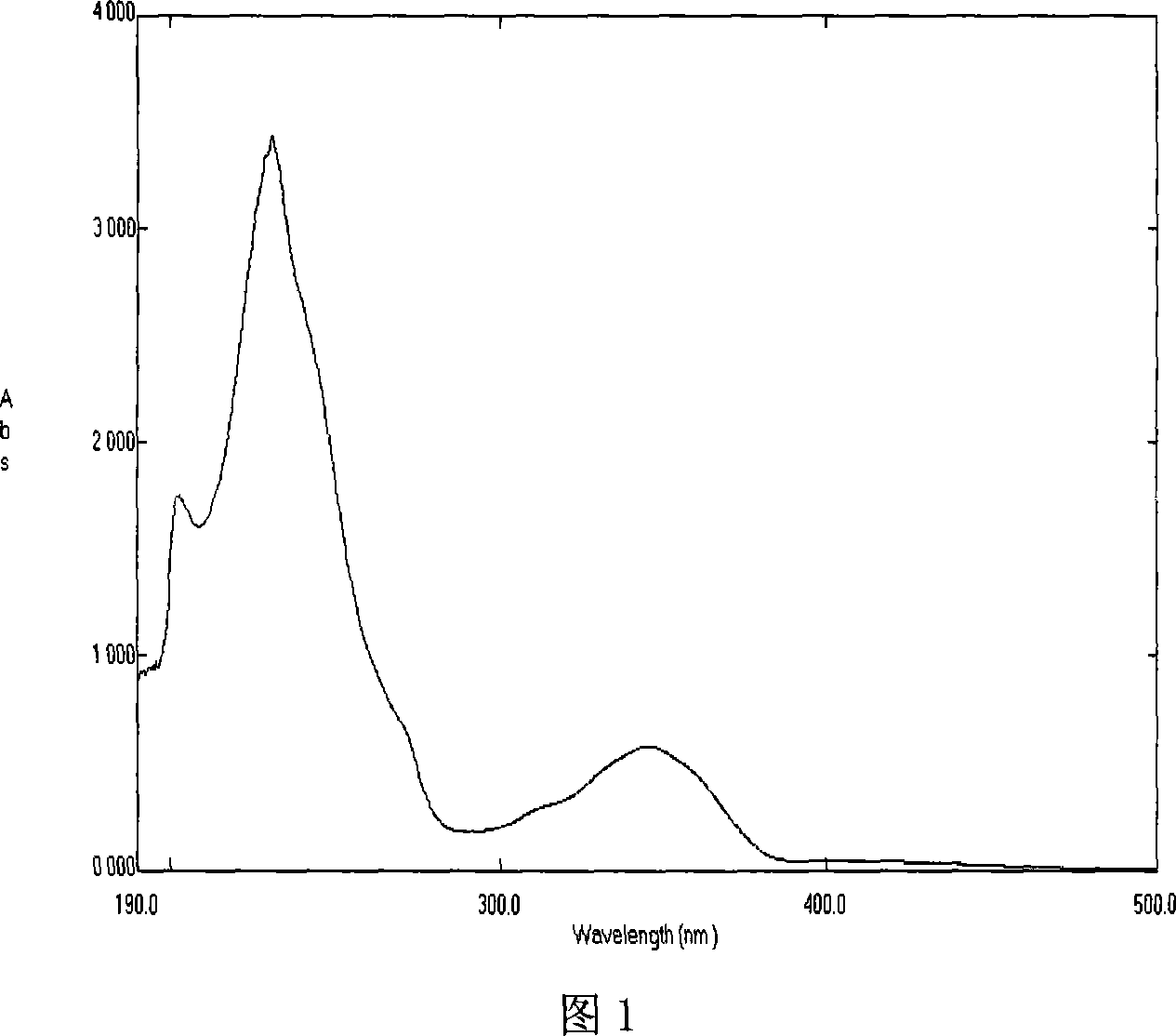
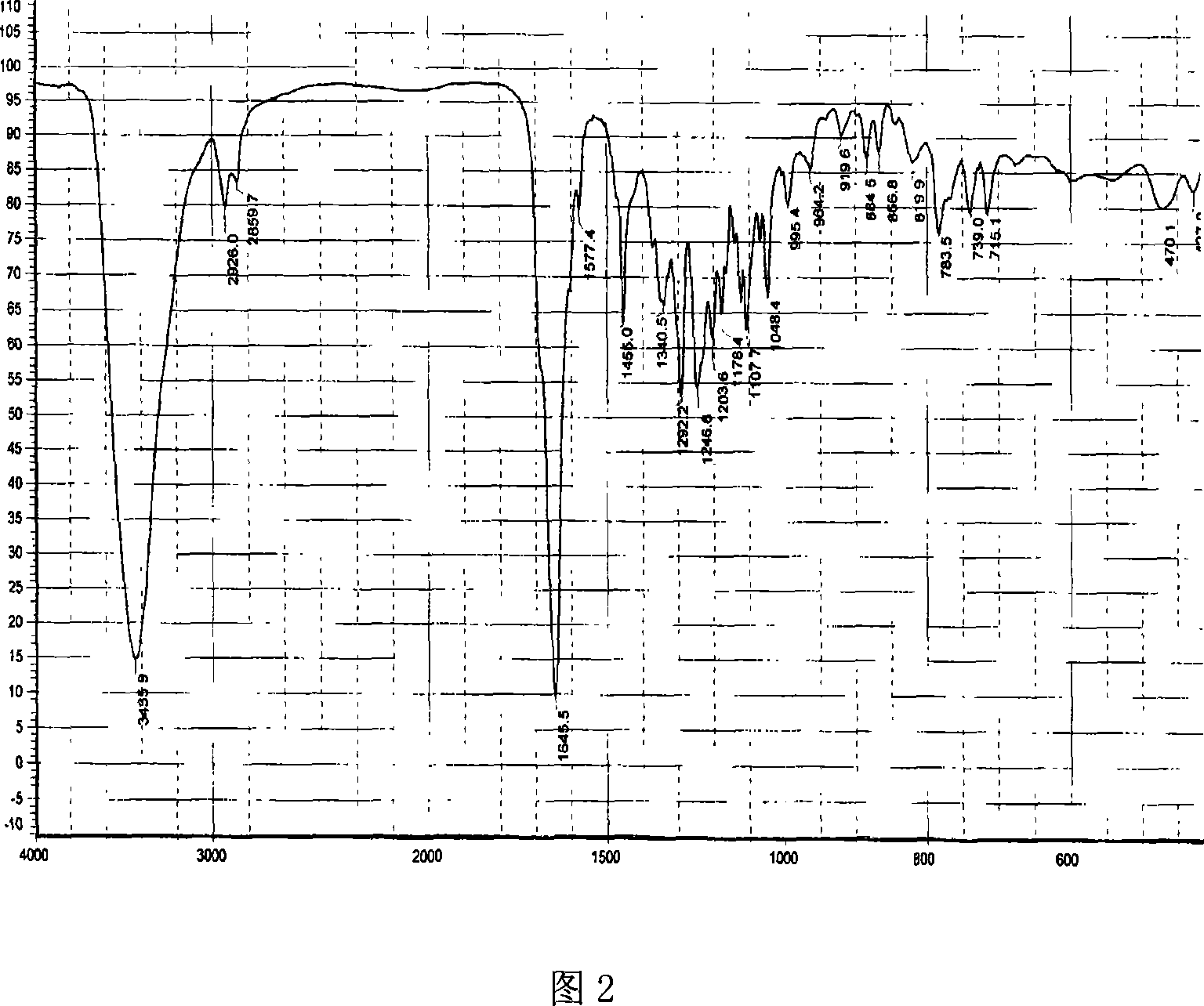
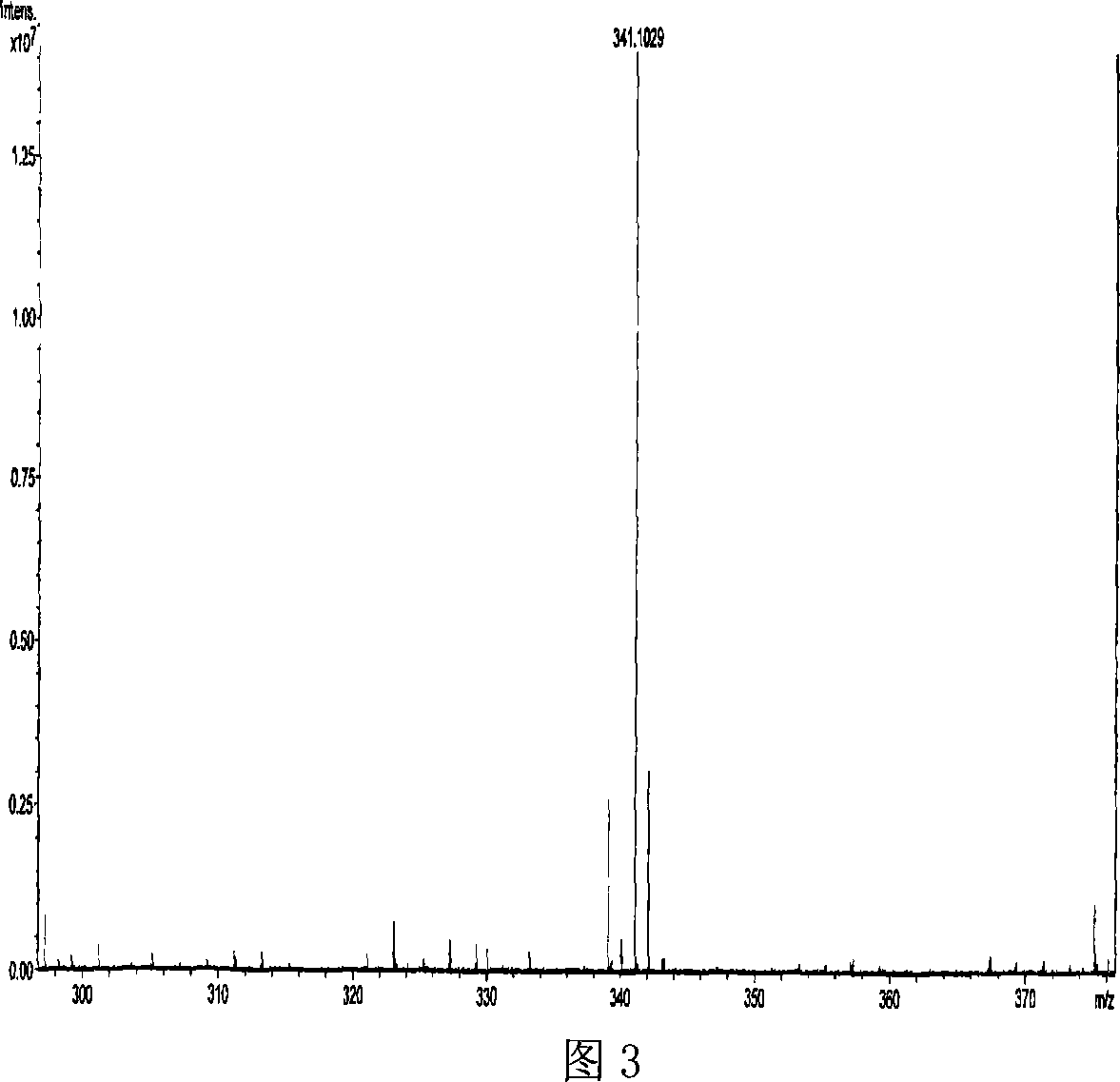
Novel antibiotic Chemomycin A, B, C, D and preparation method thereof
A technology of carmomycin and antibiotics, which is applied in the field of preparation of anti-tumor drugs, and achieves a significant anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] : the fermentation of carmomycin B, C, D
[0057] (2% soluble starch, 1% KNO 3 , 0.05% NaCl, 0.05% K 2 HPO 4 ,, 0.001% FeSO 4 ·7H 2 O, 0.05% MgSO 4 ·7H 2 O, 2% agar, pH7.0) Nocardia MediterraneiVar.Kanglensis 1747-64 bacteria were inoculated in the seed medium (2% glucose, 1% soybean powder, 0.5% yeast powder, 0.5% CaCO 3 , pH6.5) 50ml / 250ml shake flask, cultivated on a rotary shaker at 28°C for 48 hours, then transferred to fermentation medium (3% glucose, 1% peanut powder, 1% yeast powder, 0.5% peptone, 0.1% CaCO 3 ,, pH6.5) 100ml / 500ml Erlenmeyer flask, cultured on a reciprocating shaking shaker at 28°C for 72 hours, and harvested a total of 20L of fermentation broth.
Embodiment 2
[0058] : the extraction of carmomycin B, C, D
[0059] Use 2N HCl to adjust the pH of the fermented liquid to 3-4, put filter paper in the Buchner funnel and filter with suction to remove hyphae, etc., to obtain the supernatant fermented liquid. Extract three times with 1 / 3 volume of ethyl acetate of the fermentation broth, combine the ethyl acetate obtained by the three extractions, add anhydrous sodium sulfate to dry and dehydrate, let stand for 3-4 hours, concentrate under reduced pressure to obtain the extract.
Embodiment 3
[0060] : separation of carmomycin B, C, D
[0061] Above gained medicinal extract is carried out preliminary separation with chloroform: methanol gradient elution earlier through silica gel column chromatography, and elution gradient is respectively chloroform: methanol=100: 1 (202mL in total), chloroform: methanol=20: 1 (300mL in total), Chloroform:methanol=9:1 (200mL in total), collect each fraction, combine and concentrate the components containing the target compound-camomycin B; then use SephadexLH-20 to further separate the components containing the target compound, elute The solvent is methanol, the flow rate is 1mL / 3 minutes, and 350mL of methanol is shared. The components in the eluate are collected, and the components containing the target compound (camomycin B) are combined and concentrated; then the polyamide membrane is used to prepare (20×20cm, chloroform:methanol:water=9:1:0.5), collect R f =0.65 green fluorescent band (camomycin B), concentrated after methanol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com