Hybrid molecules qa, wherein q is an aminoquinoline and a is an antibiotic or a resistance enzyme inhibitor, their synthesis and their uses as antibacterial agent
An aminoquinoline and antibiotic technology, applied to a hybrid QA molecule in which Q is an aminoquinoline and A is an antibiotic residue, which can solve the problem of not showing the special activity of the aminoquinoline substituent.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The invention also relates to processes for their preparation, to their various uses, to pharmaceutical compositions containing them, and to methods of therapeutic treatment. These new molecules could also be used as antimicrobial agents.
[0030] According to a first aspect, the present invention provides hybrid aminoquinoline-antibiotic compounds, characterized in that they have the following general formula (I):
[0031] Q-(Y 1 ) p -(U) p’ -(Y 2 ) p” -A (I)
[0032] in:
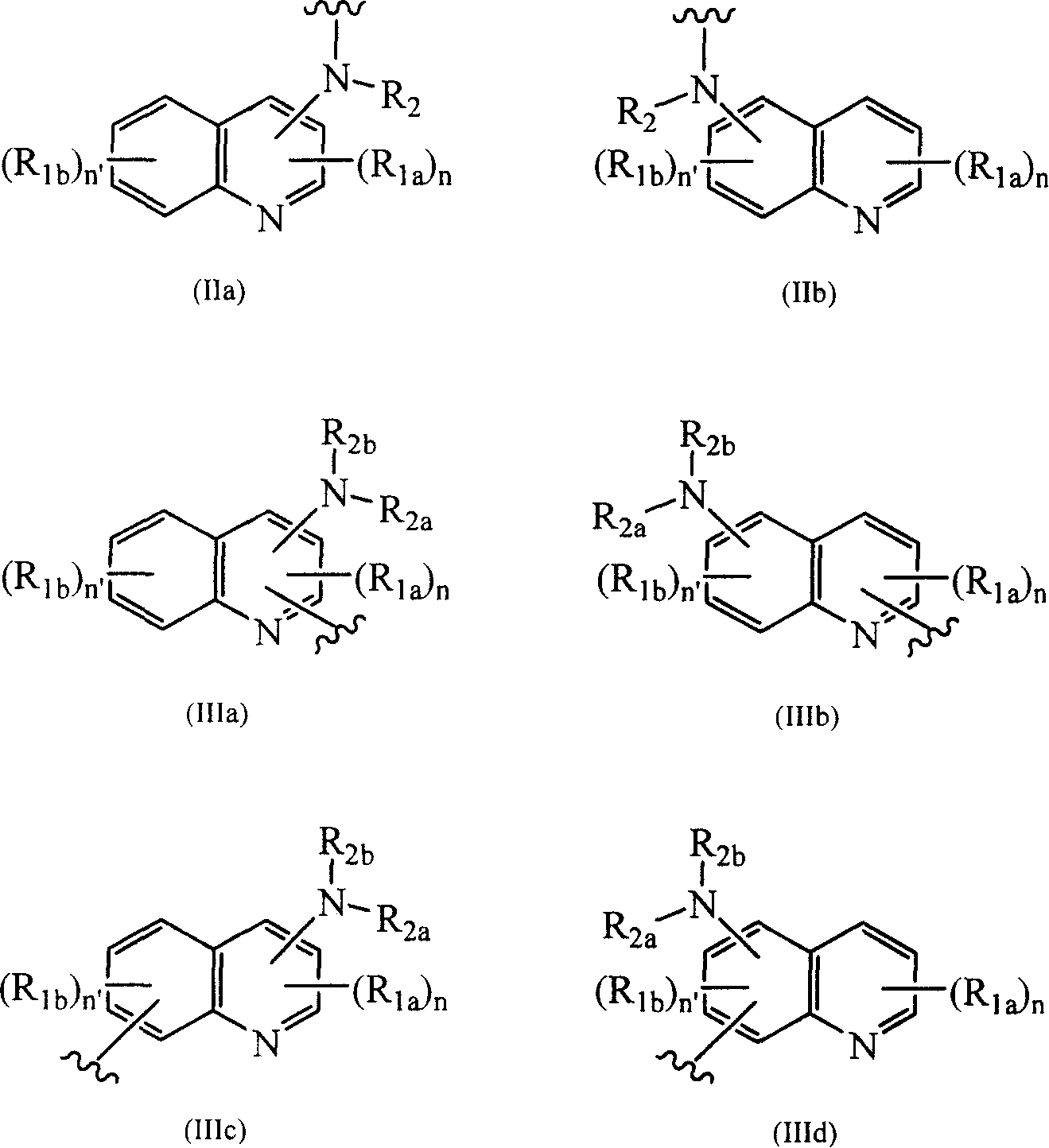
[0033] -Q represents an aminoquinoline having the following formula (IIa), (IIb), (IIIa), (IIIb), (IIIc) or (IIId):
[0034]
[0035] In the above formula:
[0036] - symbol Indicates other fragments, such as Y 1 , or U, or Y 2 , or a fixed bit of A;
[0037] - n and n' represent 0, 1, 2 or 3 independently of each other;
[0038] -R 1a and R 1b denotes the same or different substituent(s) occupying any position and denotes a substituent selected from the group cons...
Embodiment 1-4
[0505] Examples 1-4 below illustrate the preparation of hybrid molecules of the quinoline-penicillin family
Embodiment 1
[0506] Embodiment 1: the preparation of quinoline-penicillin, reference number PA 1007
[0507] (2S, 5R, 6R)-6-{[1-(7-chloro-quinolin-4-yl)-piperidine-4-carbonyl]-amino}-3,3-dimethyl-7-oxo - 2,2-dimethyl-propionyloxymethyl 4-thio-1-aza-bicyclo[3.2.0]heptane-2-carboxylate.
[0508]
[0509] 1.1. 1-(7-Chloro-quinolin-4-yl)-piperidine-4-carboxylic acid.
[0510] A mixture of 4,7-dichloroquinoline (17.4 g, 0.09 mol), isonipecotic acid (23.8 g, 0.18 mol) and phenol (46.3 g, 0.49 mol) was heated at 120°C under magnetic stirring for 24 hours. After returning to room temperature, the reaction medium is diluted with 400 ml of ethyl acetate, filtered through sintered glass and the resulting precipitate is washed with water. Next, the precipitate was recrystallized by thermal dissolution (100° C.) in 300 ml of 10% (w / v) carbonate-containing water and precipitated at 0° C. by adding 2M aqueous hydrochloric acid until pH 5. The precipitate formed was filtered off, washed successively...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com