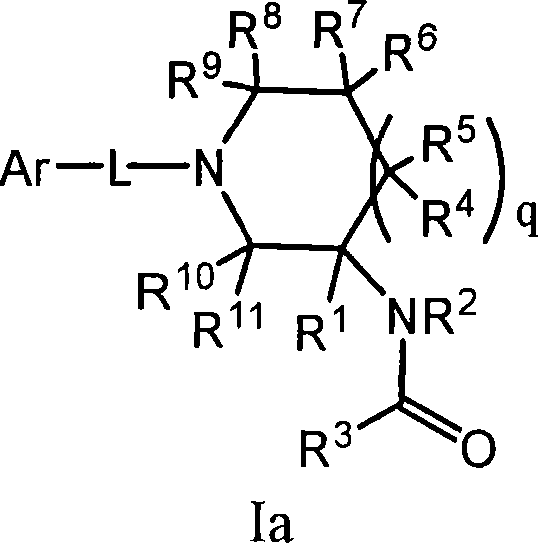
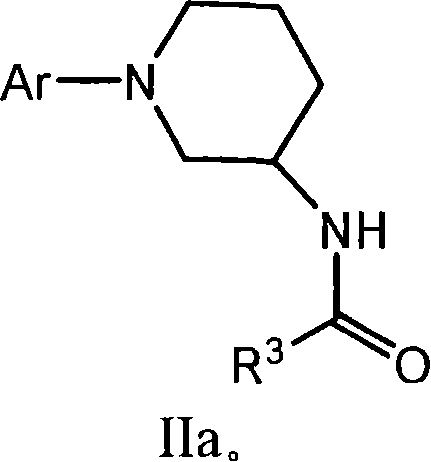
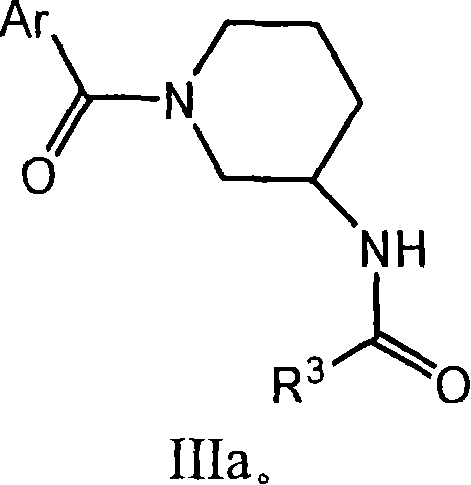
Amido compounds and their use as pharmaceuticals
A technology of compounds and prodrugs, applied in the field of modulators of type 1 11-β hydroxysteroid dehydrogenase and/or mineralocorticoid receptors, which can solve problems such as increased blood pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0393]
[0394] N-(3R)-1-[(3-Chloro-2-methylphenyl)sulfonyl]piperidin-3-ylcyclohexanecarboxamide
[0395] Step 1: N-[(3R)-piperidin-3-yl]cyclohexanecarboxamide hydrochloride
[0396] Cyclohexanecarbonyl chloride (70.0 μL, 0.515 mmol) was added to (3R)-tert-butyl 3-aminopiperidine-1-carboxylate (100.0 mg, 0.499 mmol) and potassium carbonate (150 mg, 2.1 eq.) at room temperature in a mixture in acetonitrile (3.0 mL). The reaction mixture was stirred at room temperature for 1 hour and filtered. The filtrate was concentrated under reduced pressure. The residue was treated with 4.0 M hydrochloric acid in 1,4-dioxane (2.0 mL) at room temperature for 1 hour. The solvent was evaporated under reduced pressure to give the product which was used in the next reaction without further purification.
[0397] Step 2: N-(3R)-1-[(3-Chloro-2-methylphenyl)sulfonyl]piperidin-3-ylcyclohexanecarboxamide
[0398]N-[(3R)-piperidin-3-yl]cyclohexanecarboxamide hydrochloride (12.3 mg, 50.0 mol) i...
Embodiment 2
[0400]
[0401] N-(3R)-1-[(2-nitrophenyl)sulfonyl]piperidin-3-ylcyclohexanecarboxamide
[0402] This compound was prepared by methods similar to those of Example 1. LCMS: (M+H) + = 396.0.
Embodiment 3
[0404]
[0405] N-[(3R)-1-(2-naphthalenesulfonyl)piperidin-3-yl]cyclohexanecarboxamide
[0406] This compound was prepared by methods similar to those of Example 1. LCMS: (M+H) + = 401.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com