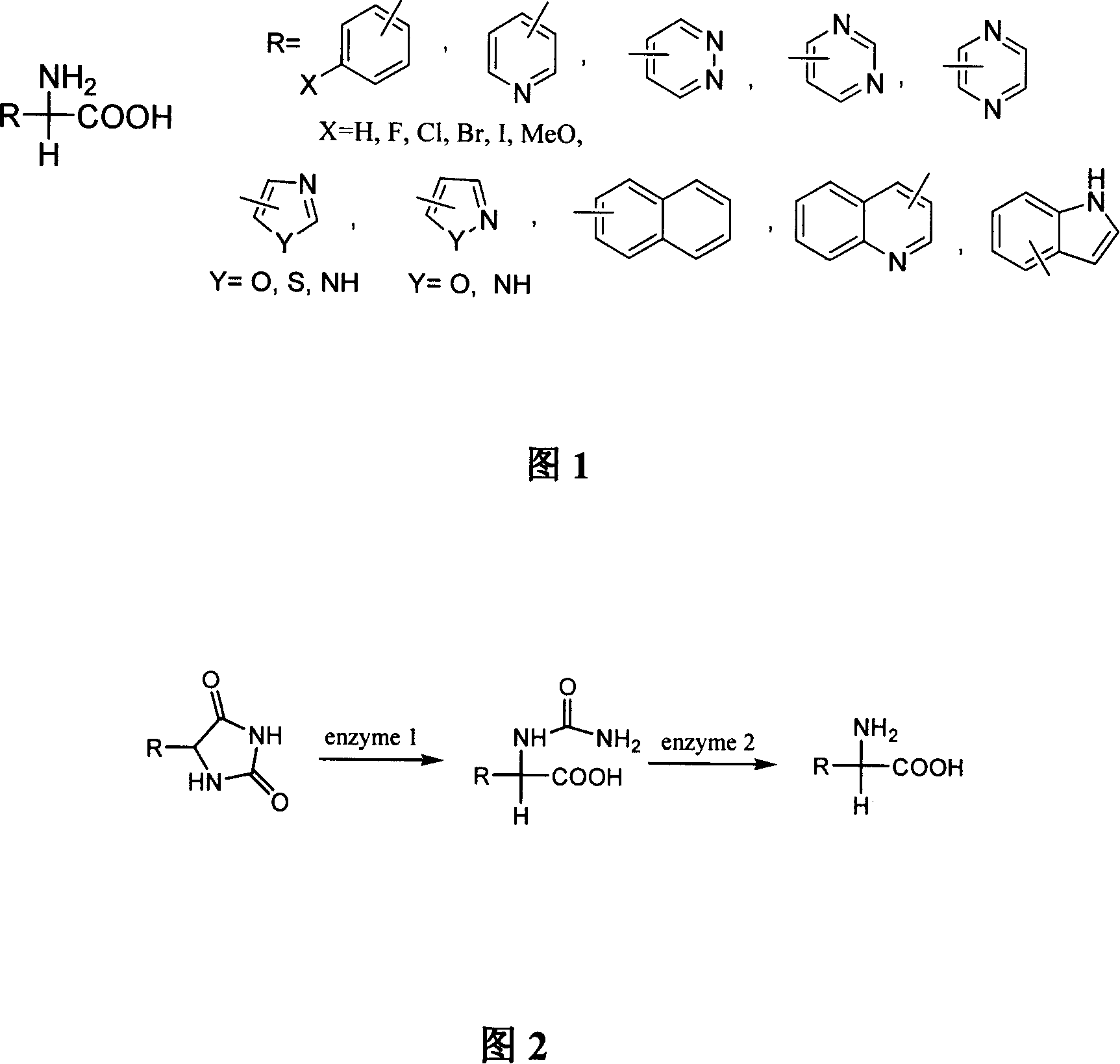
Method for synthesizing D-arylglycine by using heterogeneous enzyme to catalytically hydrolyzing 5-arylhydantoin
A technology for catalytic hydrolysis of arylglycine, applied in the field of heterogeneous enzyme catalyzed hydrolysis of 5-arylhydantoin to synthesize D-arylglycine, can solve the problems of low solubility, large reaction volume, high energy consumption, etc. clip effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1, the preparation of D-phenylglycine
[0060] In a 10L enzyme-catalyzed reaction tank, first add 7kg of water, then add 1kg of 5-phenylhydantoin, add hydrochloric acid or sulfuric acid to adjust the pH to 6.8, and then add wet enzyme activity of 0.62U / ml under stirring conditions The preparation solution is about 0.3L, the temperature is controlled at 28°C, and the reaction is stirred. Samples were taken at intervals of 6 hours, and the reaction was detected by liquid chromatography. When the reaction lasted for about 8 hours, D-phenylglycine crystals began to precipitate, and the product concentration in the reaction solution was basically constant at 2.45%. When the substrate 5-phenylhydantoin cannot be detected in the reaction system and the N-carbamoylglycine is below 0.25%, the reaction is basically completed, and the conversion rate is above 99.6%. The crude crystals obtained by sieving and separation can be recrystallized to obtain 446.2g of qualifie...
Embodiment 2
[0066] Embodiment 2, preparation of D-3-pyridine glycine
[0067] In a 10L enzyme-catalyzed reaction tank, first add 6.5kg of water, add 1kg of 5-(3-pyridyl)hydantoin under stirring conditions, adjust the pH to 6.5, and then add wet enzyme activity of 0.58U / ml Enzyme preparation solution is about 0.4L, stirred and reacted at 32-36°C. Samples were taken at intervals of 8 hours, and the reaction was detected by liquid chromatography. When the reaction lasted for about 9 hours, D-pyridine glycine crystals began to precipitate. When the substrate 5-(3-pyridyl)hydantoin cannot be detected in the reaction system and the N-carbamoylpyridine glycine is below 0.20%, the reaction is basically completed, and the conversion rate is about 99.5%. The coarse crystals obtained by sieving and separation can be recrystallized to obtain 391.6 g of qualified product D-3-pyridine glycine; the material that has passed through the sieve is obtained by membrane filtration, concentration and other re...
Embodiment 3
[0069] Embodiment 3, the preparation of D-2-naphthylglycine
[0070] In a 10L enzyme-catalyzed reaction tank, first add 7.5kg of water, add 0.8kg of 5-(2-naphthyl)hydantoin under stirring conditions, adjust the pH to 7.0, and then add wet enzyme activity to 0.65U / ml The enzyme preparation solution is about 0.4L, and the reaction is stirred at 30-40°C. Samples were taken at intervals of 8 hours, and the reaction was detected by liquid chromatography. When the reaction was carried out to about 7 hours, D-2-naphthylglycine crystals began to precipitate. When no 5-(2-naphthyl)hydantoin and corresponding intermediates can be detected in the reaction system, the reaction is basically finished, and the conversion rate is about 99%. The coarse crystals obtained by sieving and separation can be recrystallized to obtain 327.3g of qualified product D-2-naphthylglycine; the material that has passed through the sieve is obtained by membrane filtration, concentration and other refining pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com