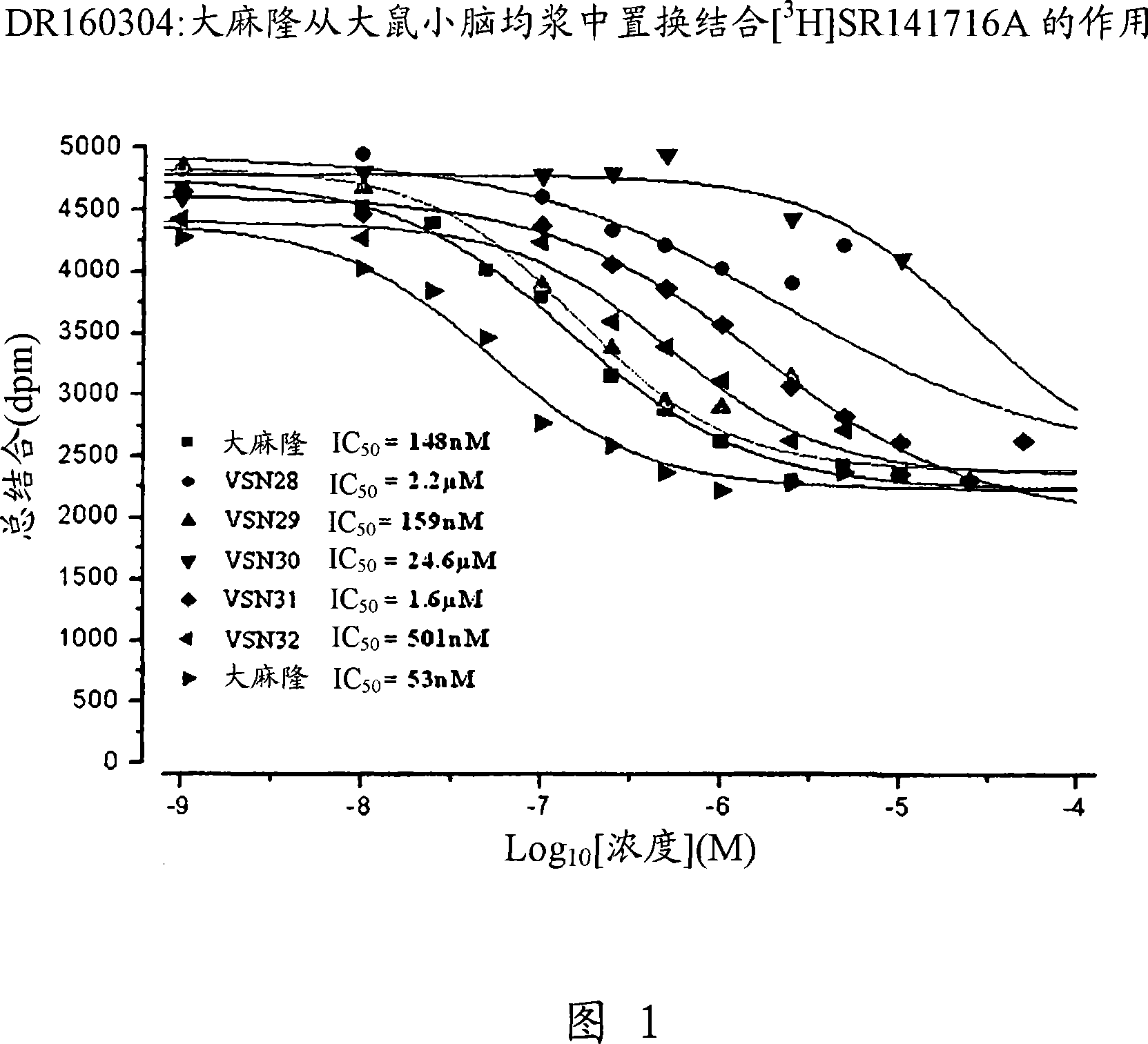
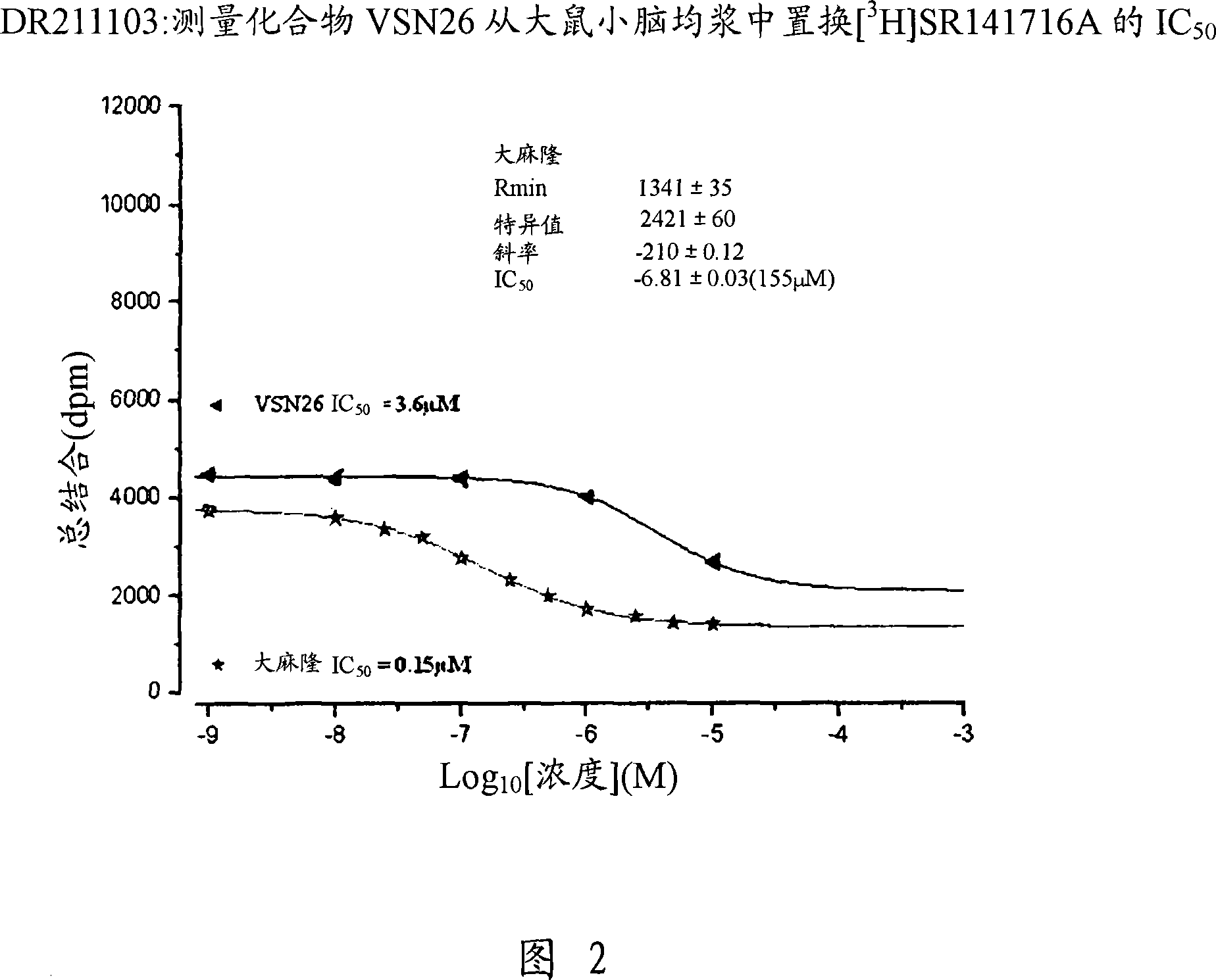
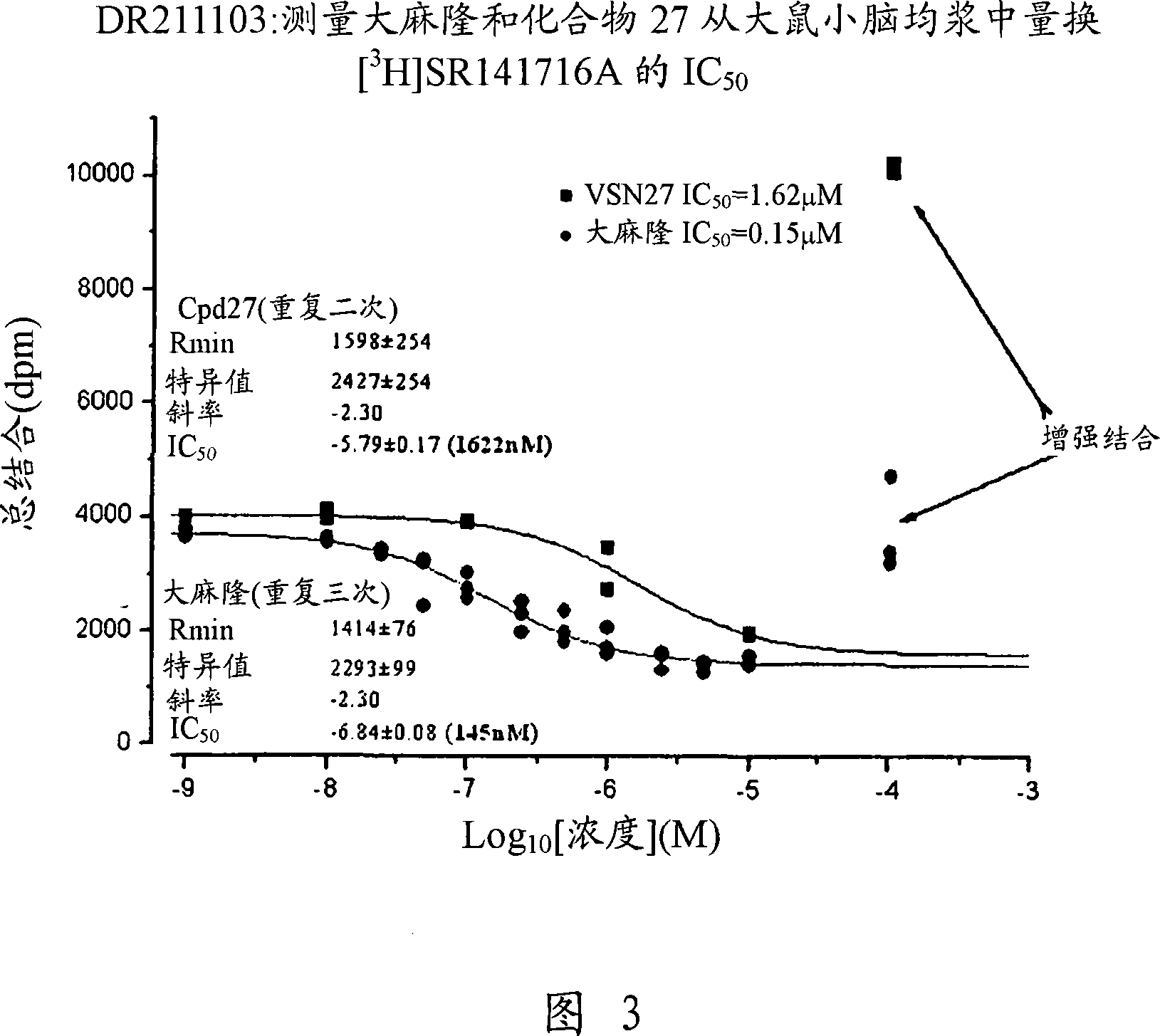
Modulator
A compound and alkyl technology, applied in the field of regulating cannabinoid receptors, to achieve the effect of reducing lipophilicity and improving water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0234] overview
[0235] Unless noted, all starting materials were either commercially available or reported in previous literature. Solvents and reagents were used without further purification, except tetrahydrofuran (THF), which was dried over sodium. The reaction was monitored by thin layer chromatography (TCL) on precoated silica gel plates (Kieselgel 60F254, Merck). Unless otherwise noted, use Still 36 Purification was carried out by flash chromatography using a column packed with silica gel (particle size 40-63 μM, Merck). Recorded on a Bruker AMX-300 spectrometer 1 H and 13 CNMR spectrum. Chemical shifts are reported in ppm. Coupling constants are in Hz. Mass spectra were recorded on a VG ZAB SE spectrometer (ESP, FAB) or a Micromass Quattro electrospray liquid crystal mass spectrometer (LCMS). Using CEM Focused Microwave TM The Synthesis System performs certain Suzuki coupling reactions.
[0236] synthesis
[0237] Compounds of formula I were synthesized by...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com