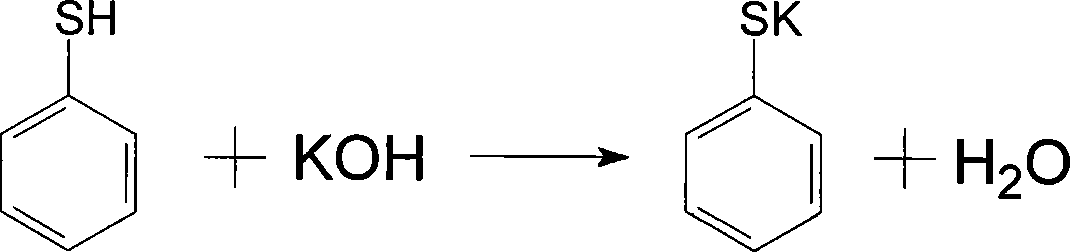
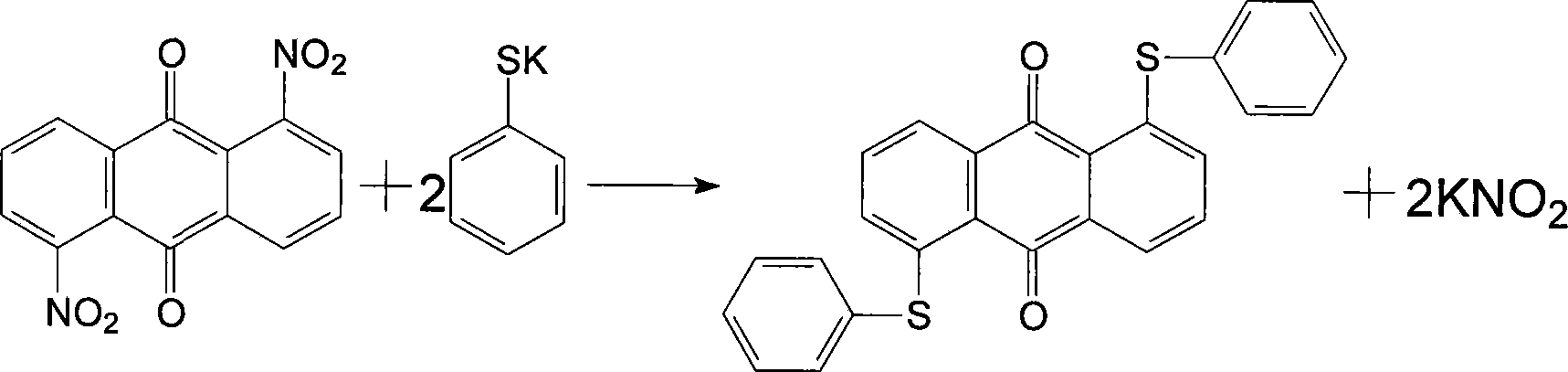
Novel technique for synthesizing solvent yellow 189
A new process and solvent technology, which is applied in the new process field of solvent yellow 189 synthesis, can solve the problems of large fluctuations in product appearance and content, high production costs, and high production costs of solvent yellow 189
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] In a 250ml four-necked flask, add 100ml of dimethylformamide, 30g of 1,5-dinitroanthraquinone, 24g of thiophenol, and 12g of potassium hydroxide. After the addition, slowly raise the temperature to 50°C for about one hour and keep it warm. React for two hours, after the chromatographic detection of 1,5-nitroanthraquinone disappears, use a cold water bath to cool down to 25°C, keep stirring at this temperature for one hour, filter, and wash the filter cake with dimethylformamide containing 20% water. Wash it with hot water at about 80°C, and dry the filter cake to obtain 41g of finished product, with a content of 99.7% and a yield of 96%.
Embodiment 2
[0017] In a 250ml four-neck flask, add 120ml of dimethylformamide, 30g of 1,5-dinitroanthraquinone, 24g of thiophenol, and 12g of potassium hydroxide. After the addition, slowly raise the temperature to 50°C for about one hour to keep the temperature React for two hours, after the chromatographic detection of 1,5-dinitroanthraquinone disappears, use a cold water bath to cool down to 25°C, keep stirring at this temperature for one hour, then filter, and wash the filter cake with dimethylformamide containing 20% water Then wash it with hot water at about 80°C, and dry the filter cake to obtain 40 g of finished product, with a content of 99.7% and a yield of 93.7%.
Embodiment 3
[0019] In a 250ml four-neck flask, add 100ml of dimethylacetamide, 30g of 1,5-dinitroanthraquinone, 24g of thiophenol, and 12g of potassium hydroxide. After 1,5-dinitroanthraquinone disappears, use a cold water bath to cool down to 25°C, keep stirring at this temperature for one hour and then filter, wash the filter cake with dimethylacetamide containing 20% water, and then wash it with about 80°C After washing with hot water, the filter cake was dried to obtain 41.5 g of finished product with a content of 99.4% and a yield of 97.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com