Hardening accelerator, hardenable resin composition, and electronic component device
一种固化促进剂、固化性树脂的技术,应用在固化性树脂组合物领域,能够解决难获得流动性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0228] Hereinafter, the present invention will be specifically described according to the embodiments, but the scope of the present invention is not limited to the embodiments shown below. Needless to say, as long as it does not depart from the gist of the present invention, it is possible to carry out various changes sex.
[0229] [Preparation of curing accelerator]
[0230]Before preparing curable resin composition, the compound used as a hardening accelerator in each Example of this invention was prepared according to synthesis examples 1-9. Furthermore, in each synthesis example, 4-triphenylphosphonium-base (phosphonio) phenoxide, 2-triphenylphosphonium-base phenoxide, 3-triphenylphosphonium-base phenoxide, 2,6-Dimethyl-4-triphenylphosphonium-base phenoxide, 3-three-p-tolylphosphonium-base phenoxide, and cyclohexyldiphenylphosphonium-base phenoxide are all made by patent-opened Synthesized by the method described in the 2004-156036 bulletin.
[0231] In addition, the an...
Synthetic example 1
[0239] 10.9 g (30.8 mmol) of 4-triphenylphosphorylphenoxide was dispersed (partially dissolved) in 100 ml of acetone, and 10.0 g (46.2 mmol) of diphenylsilanediol was added to the solution while stirring. The light yellow 4-triphenylphosphonium phenate powder in the solution will slowly turn white. Considering that diphenylsilanediol is soluble in acetone, this change may be due to the reaction of partially dissolved 4-triphenylphosphinylphenate with diphenylsilanediol and gradually being consumed , so as to precipitate out with the salt of 4-triphenylphosphonium-base phenoxide and diphenylsilanediol. After the reaction mixture was stirred at room temperature for 12 hours, it was filtered and dried to obtain 16.3 g of a white solid product.
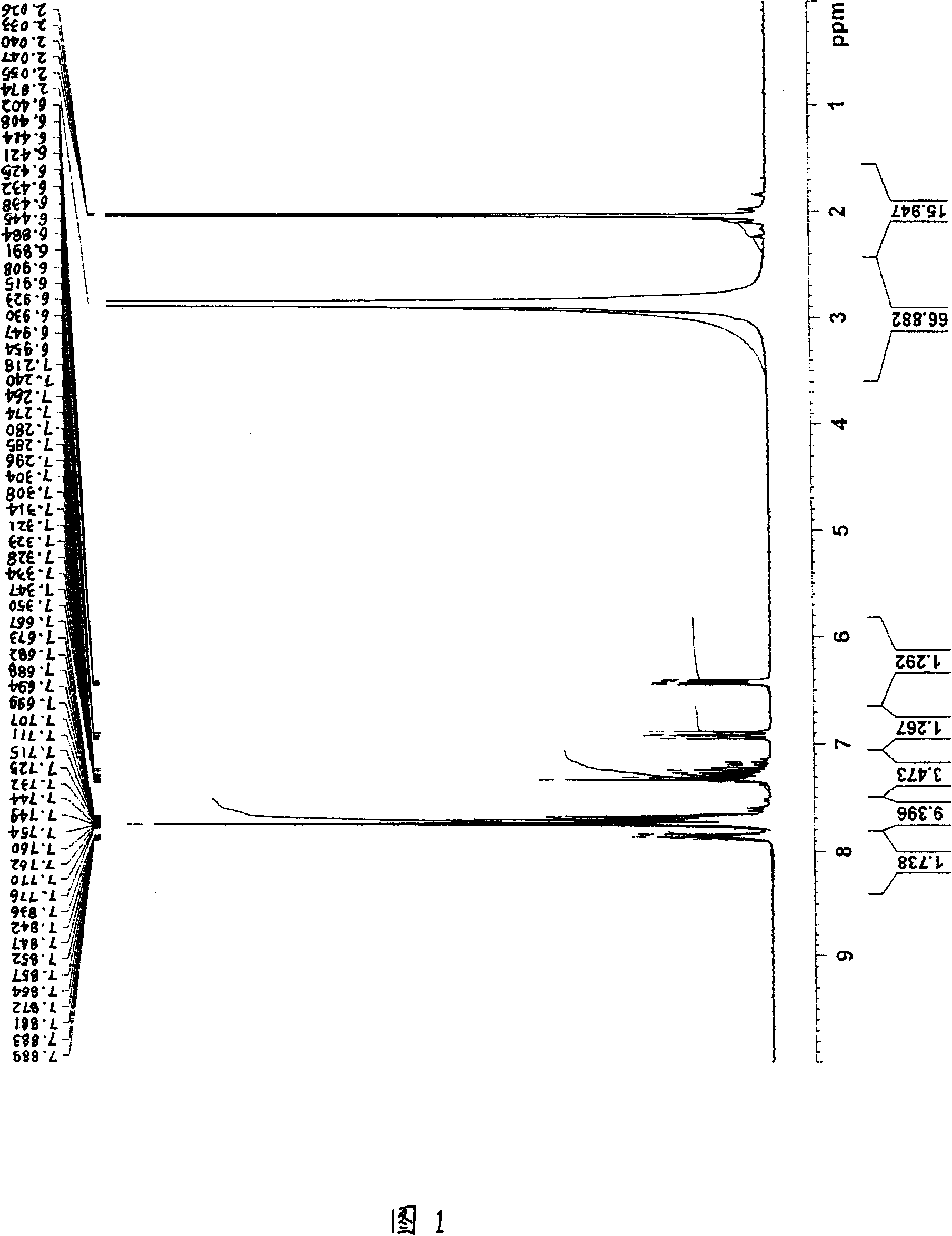
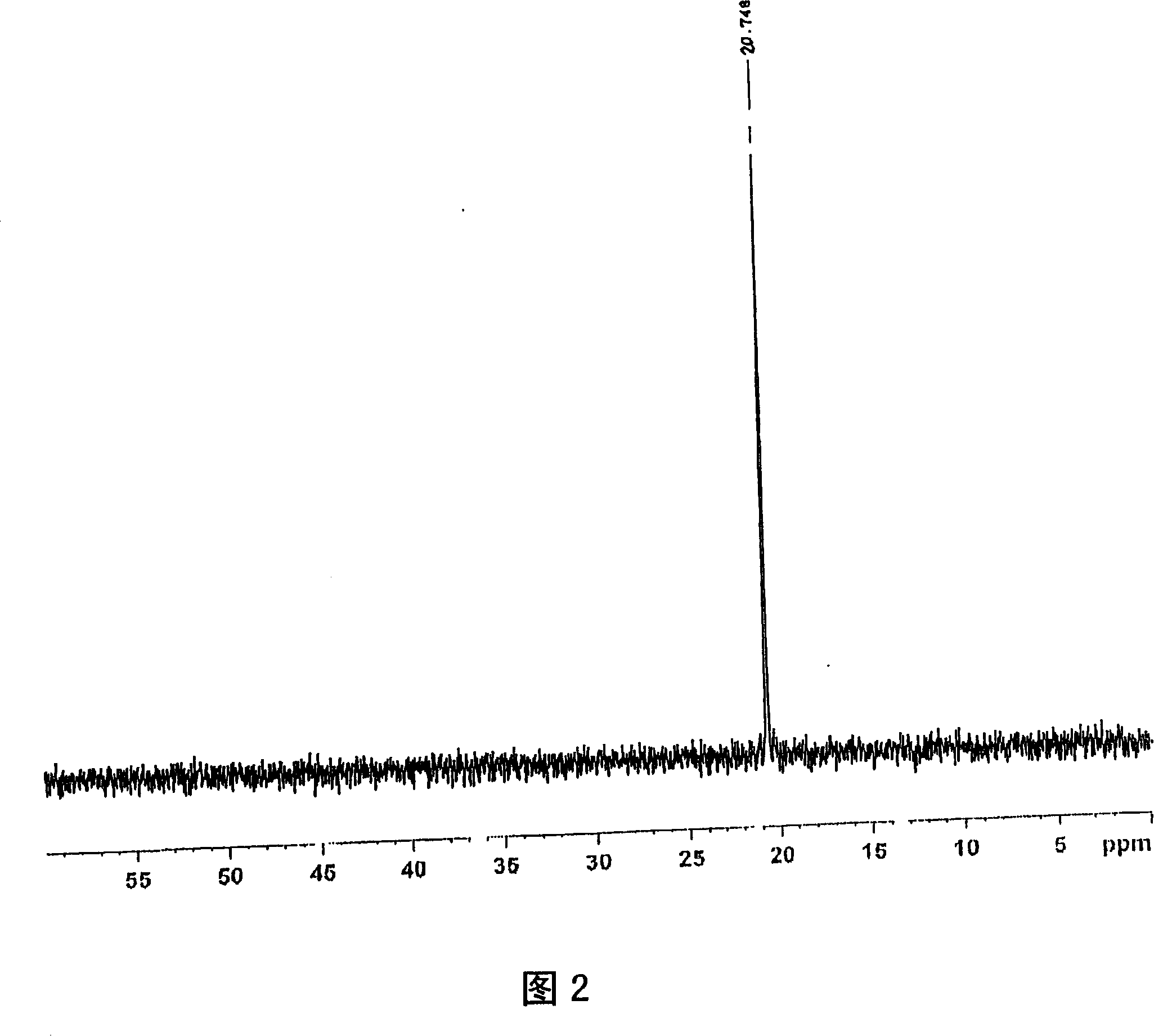
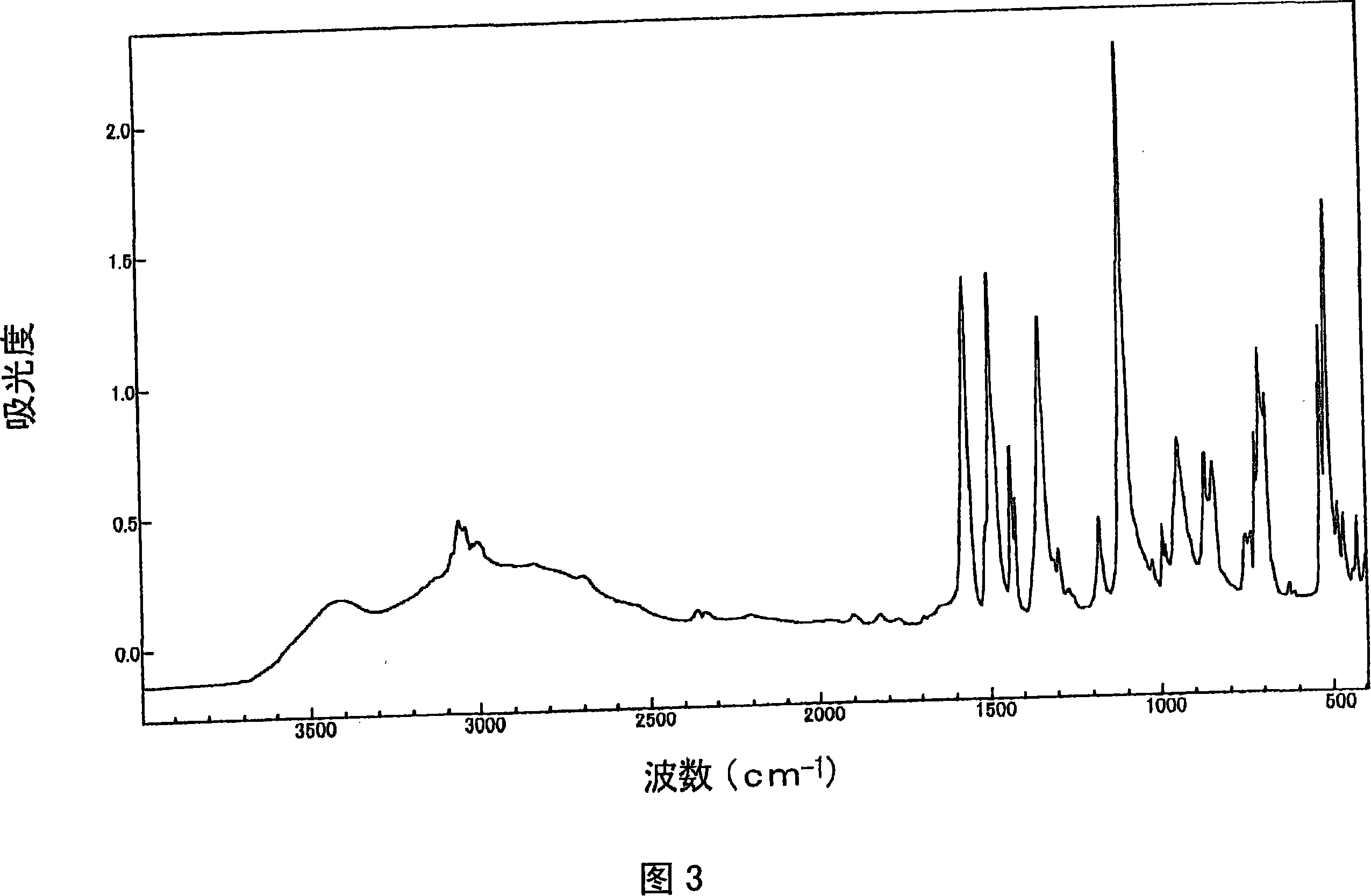
[0240] For the obtained product, the 1 H-NMR determination, 31 As a result of P-NMR measurement (measurement in deuterium methanol) and IR measurement, the spectra shown in FIGS. 1 to 3 were respectively obtained. As a result of ident...
Synthetic example 2
[0244] 30.0 grams (84.6 mmol) of 4-triphenylphosphine-based phenoxide were dispersed (partially dissolved) in 60 ml of acetone and 30 ml of distilled water, and while stirring in the solution, 18.3 g (84.6 mmol) of diphenylsilanediol was added. ). Accompanied by the addition of diphenylsilanediol, 4-triphenylphosphine-base phenoxide will dissolve first, and immediately separate out white powder. The reaction mixture was stirred at room temperature for 30 minutes, and after distilling off about 30 ml of acetone with an evaporator, it was filtered and dried to obtain 43.5 g of a white solid product.
[0245] For the obtained product, the 1 H-NMR determination, 31 As a result of P-NMR measurement (measurement in deuterium methanol) and IR measurement, the same spectrum as in Synthesis Example 1 was obtained. Therefore, it was confirmed that the product had the structure represented by the above-mentioned formula (XX) similarly to Synthesis Example 1 (hereinafter referred to as...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com