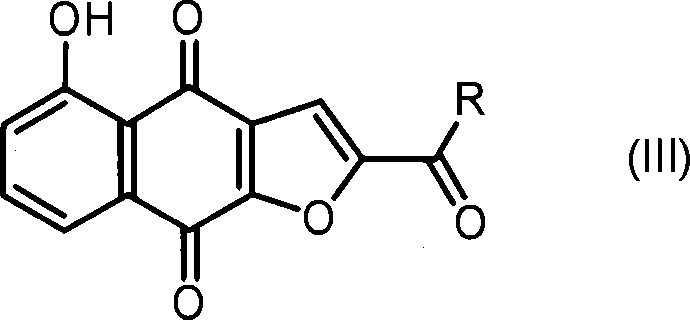
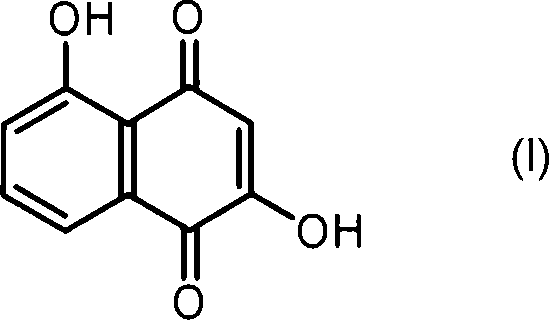
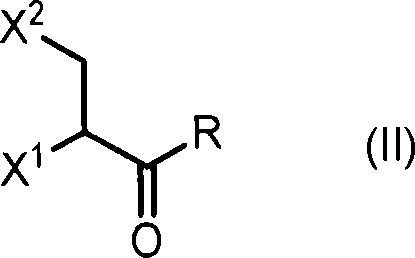
Anticancer compound, intermediate therefor, and processes for producing these
A technology for compounds and racemates, applied in the field of anticancer compounds and their intermediates and production, can solve the problems of low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Preparation of 2-Dimethyljuglone
[0072]
[0073] To a solution of 5-hydroxynaphthalene-1,4-dione (also known as juglone) (171 mg, 1 mmol) in toluene (5 mL) at -20°C was added dimethylamine (0.75 mL, 2.0M solution in tetrahydrofuran, 1.5 mmol). The mixture was stirred at -20°C for 1 hour. Dimethylamine (0.75 mL, 2.0 M in tetrahydrofuran, 1.5 mmol) was then added thereto, the mixture was stirred at -20°C for 30 minutes, and then the solvent was evaporated in vacuo. The residue was purified by silica gel column chromatography (chloroform / ethyl acetate=20 / 1 (V / V)) to separate 2-dimethylaminojuglone (87.2 mg, 40%) and 3-dimethylaminojuglone (28.8 mg, 13%).
[0074] 2-Dimethylaminojuglone
[0075] Melting point: 147 to 148°C
[0076] 1 H-NMR (CDCl 3 ): δ 3.25 (s, 6H), 5.72 (s, 1H), 7.20 (dd, 1H, J = 1.2, 8.3 Hz), 7.45-7.51 (m, 2H), 13.0 (s, 1H).
[0077] 3-Dimethylaminojuglone
[0078] 1 H-NMR (CDCl 3 ): δ 3.23 (s, 6H), 5.84 (s, 1H), 7.15 (dd, 1H, J = 3.7, 6.1...
Embodiment 2
[0080] The reaction was carried out in a manner similar to that described in Example 1, except that -40°C was substituted for -20°C to obtain 2-juglone (104 mg, 48%) and 3-juglone (20 mg, 10% ).
Embodiment 3
[0082] The reaction was carried out in a manner similar to that described in Example 1, except that water was used to replace tetrahydrofuran as a solvent for dimethylamine, and 0.15 ml of aqueous dimethylamine solution (50% aqueous solution, 1.5 mmol) was used at a reaction temperature of 0 ° C to obtain 2-Dimethylaminojuglone (97 mg, 45%) and 3-Dimethylaminojuglone (67 mg, 31%). The use of water instead of organic solvents makes this method more favorable in terms of environment and safety.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com