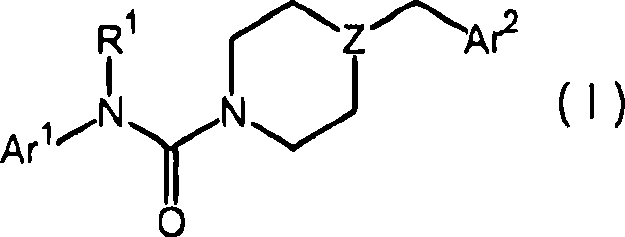
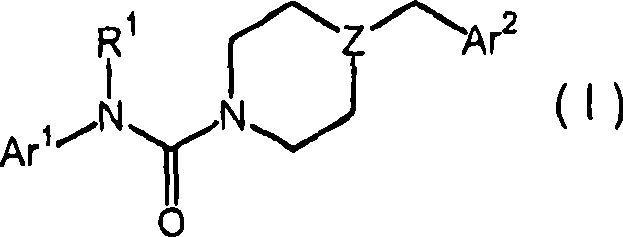
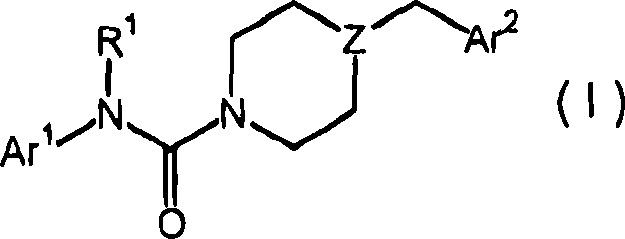
Piperazinyl and piperidinyl ureas as modulators of fatty acid amide hydrolase
A pyridinyl, pyrimidinyl technology in the field of piperazinyl urea and piperidinyl urea compounds capable of addressing undisclosed catalepsy, hypothermia, or enhanced feeding behavior
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0348] Example 1: tert-butyl 4-phenylcarbamoyl-piperazine-1-carboxylate (intermediate)
[0349]
[0350] A solution of tert-butyl piperazine-1-carboxylate (114g) in DCM (500ml) was cooled in an ice bath and treated with phenylisocyanate (65ml). After 1 hour (h), the ice bath was removed. After 15 hours, the resulting mixture was filtered and the solid was washed with dichloromethane (DCM, 2 x 100ml) to give the title compound as a white amorphous solid (95g).
Embodiment 2
[0351] Embodiment 2: Piperazine-1-carboxylic acid phenylamide (intermediate)
[0352]
[0353] with 2M HCL / Et 2 O (164ml) was treated with tert-butyl 4-phenylcarbamoyl-piperazine-1-carboxylate (50g) in MeOH (1L). After 48 hours, with Et 2 The resulting suspension was diluted with O (1 L) and filtered. with Et 2 The solid was washed with O (3 x 100ml) and dried in vacuo to give a white powder (32g). The powder was partitioned between DCM (400ml) and 10% aqueous KOH (400ml). The aqueous phase was extracted with DCM (2 x 400ml). The combined organic phases were dried (MgSO 4 ) and concentrated to afford the title compound as a white amorphous solid (26 g).
Embodiment 3
[0354] Example 3: tert-butyl 4-(4-fluoro-phenylcarbamoyl)-piperazine-1-carboxylate (intermediate)
[0355]
[0356] The title compound was prepared analogously to Example 1 using 4-fluorophenyl isocyanate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com