Flavone derivative, preparation method and application
A technology of derivatives and flavonoids, applied in the field of flavonoid derivatives and preparation, can solve problems such as visual disturbance, headache and facial flushing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
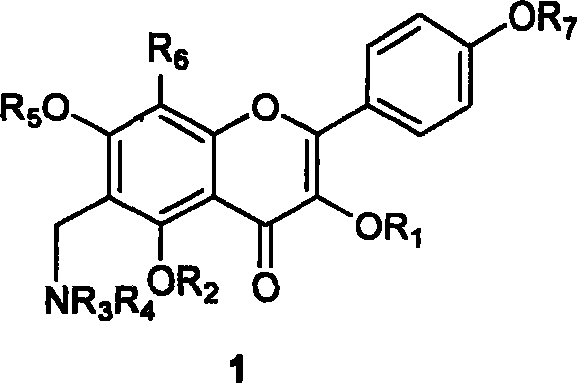
[0063] Example 1 3,7-diacetoxy-5-hydroxy-4'-methoxy-8-(3,3-dimethylpropenyl)flavone (TPN1306)
[0064]
[0065] Add 0.200 g of icariin to a 100 mL flask, dissolve it with 27 mL of anhydrous pyridine, add 0.127 mL of acetic anhydride, stir at room temperature for 3 h, then add 50 mL of saturated NaHCO 3 The aqueous solution was extracted three times with ethyl acetate (50 mL each time), the ethyl acetate layer was washed twice with saturated brine (50 mL each time), the organic phase was dried over anhydrous sodium sulfate, concentrated by filtration, and separated by silica gel column chromatography ( Eluent: petroleum ether / ethyl acetate=3:1) to obtain the target compound (0.174 g, 71%). 1 HNMR (CDCl 3 ): δ1.69 (3H, s, H-4″), 1.73 (3H, s, H-5″), 2.35 (3H, s, C 3 -OAc), 2.37 (3H, s, C 7 -OAc), 3.42 (2H, d, J=6.3Hz, H-1″), 3.90 (3H, s, OCH 3 ), 5.13 (1H, t, J=6.3Hz, H-2″), 6.58 (1H, s, H-6), 7.02 (2H, d, J=9.0Hz, H-3′, 5′), 7.52 (2H, d, J=9.0Hz, H-2', 6'), 12.15 (1H, s,...
Embodiment 2
[0066] Example 2 3,7-diacetoxy-5,4'-dimethoxy-8-(3,3-dimethylpropenyl)flavone (TPN1308)
[0067]
[0068] Add 0.010 g of the compound of Example 1 to a 5 mL flask, dissolve it in 1.1 mL of DMF, add 0.006 g of potassium carbonate and 0.014 mL of methyl iodide successively, stir at room temperature for 3 h, add 20 mL of saturated NH 4 Cl aqueous solution was extracted three times with ethyl acetate (20 mL each time), the ethyl acetate layer was washed twice with saturated brine (50 mL each time), dried over anhydrous sodium sulfate, concentrated by filtration, and separated by silica gel column chromatography ( Eluent: petroleum ether / ethyl acetate=4:1) to obtain the title compound (0.009 g, 87%). 1 H-NMR (CDCl 3 ): δ1.69 (3H, s, 3-CH 3 ), 1.73 (3H, s, 3-CH 3 ), 2.35 (3H, s, COCH 3 ), 2.36 (3H, s, COCH 3 ), 3.45 (2H, d, J=6.4Hz, H-1), 3.88 (3H, s, OCH 3 ), 3.93 (3H, s, 4'-OCH 3 ), 5.14 (1H, br t, J=6.4Hz, H-2), 6.55 (1H, s, H-6), 7.00 (2H, d, J=8.8Hz, H-3′, H-5′ ), 7....
Embodiment 3
[0069] Example 3 3,7-diacetoxy-5-ethoxy-4'-methoxy-8-(3,3-dimethylpropenyl)flavone (TPN1338)
[0070]
[0071] 0.010 gram of Example 1 compound, 1.1 mL of DMF, 0.006 gram of K 2 CO 3 , 0.004 g of KI and 0.017 mL of bromoethane were successively added into a 5 mL round bottom flask, and stirred at room temperature for 6.5 h. After the reaction was completed, 30 mL of saturated NH 4 Cl solution, extracted with EtOAc (3×30mL), the organic phase was washed with saturated brine (2×20mL), washed with anhydrous Na 2 SO 4 After drying, it was concentrated by filtration and separated by silica gel column chromatography (eluent: petroleum ether / ethyl acetate=3:1) to obtain the target compound (0.006 g, 57%). 1 H NMR (CDCl 3 ): δ1.47 (3H, t, J=6.9Hz, C 5 -OCH 2 CH 3 ), 1.69 (3H, s, H-4″), 1.76 (3H, s, H-5″), 2.33 (3H, s, C 3 -OAc), 2.43 (3H, s, C 7 -OAc), 3.58 (2H, d, J=6.6Hz, H-1″), 3.88 (3H, s, C 4 '-OCH 3 ), 4.13 (2H, q, J=6.9Hz, C 5 -OCH 2 CH 3 ), 5.20 (1H, t, J=6.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com