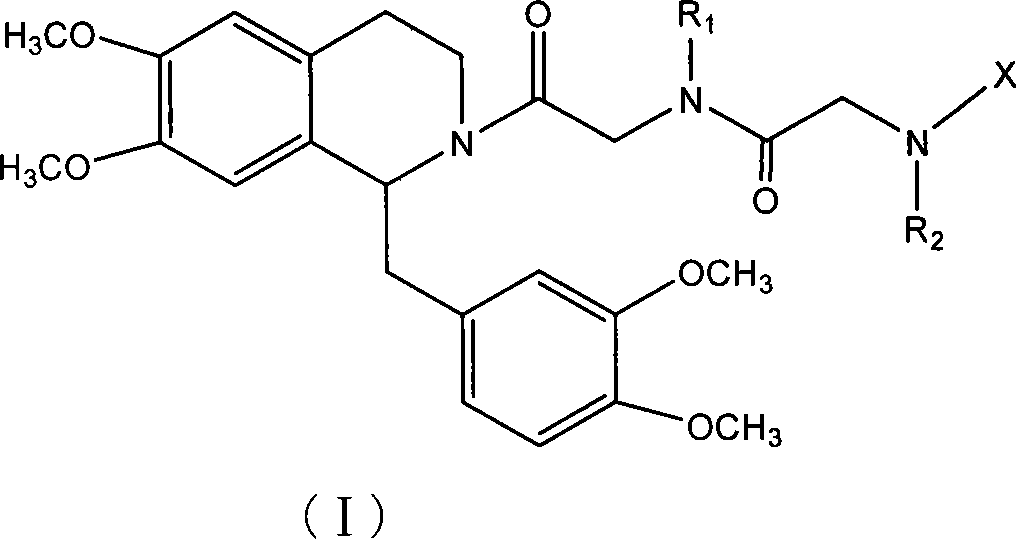
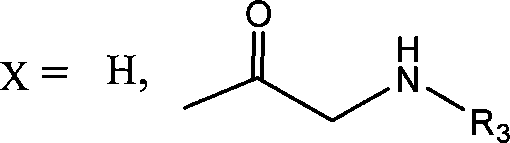
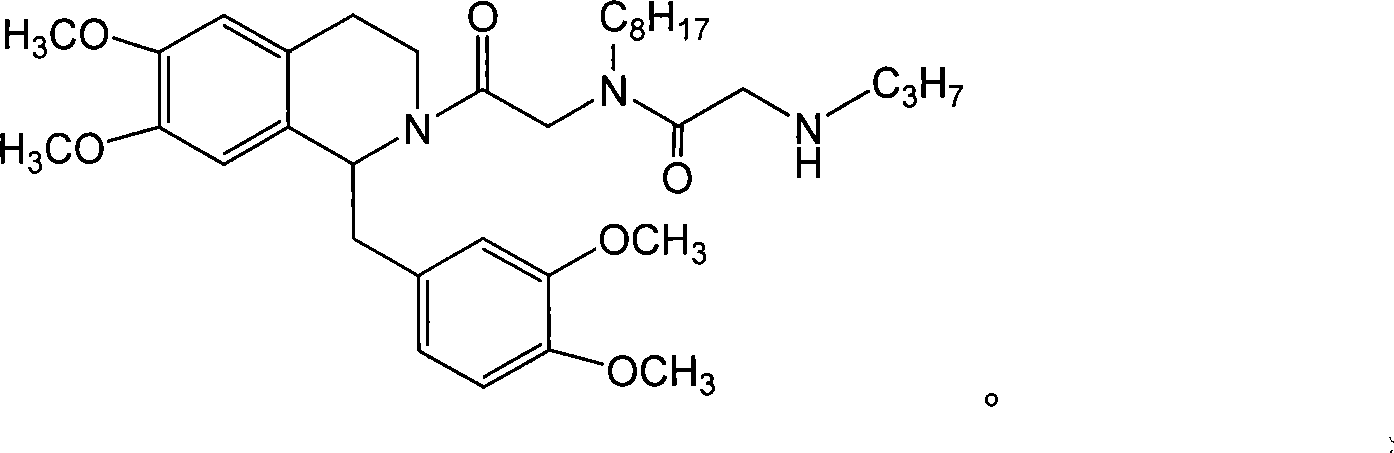
Tetrahydrochysene isoquinoline derivant, its producing method and uses of the same
A technology of tetrahydroisoquinoline and its derivatives, which is applied in the direction of pharmaceutical formulations, drug combinations, and medical preparations containing active ingredients, etc., and can solve problems such as low specificity, low reversal activity, and cardiovascular side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] N-(3,4-dimethoxy)phenethyl-(3,4-dimethoxy)phenylacetamide (II 1 ) preparation
[0085] 3,4-dimethoxyphenylethylamine (dimethoxydistilled, 140-170 / 1mmHg fraction) 18.6g (0.103mol) mixed with 3,4-dimethoxyphenylacetic acid 18.9g (0.096mol), nitrogen, Slowly heat to 190°C, water vapor is generated, all solids melt, keep warm for 3 hours, let cool to 80°C, add 150ml of chloroform to dissolve, and wash the chloroform solution with 3% hydrochloric acid, water, 3% NaOH aqueous solution, and saturated saline to medium sex, anhydrous Na 2 SO 4 Dry, evaporate to near dryness under reduced pressure, cool slightly, add 100ml of diethyl ether, shake gently, precipitate white solid, filter, dry, crude product weighs 31.9g, yield: 84%, mp120-122°C (documentation mp122.5-123°C ).
[0086] 6,7-dimethoxy-1-(3,4-dimethoxy)benzyl-3,4-dihydroisoquinoline (III 1 ) preparation
[0087] II 1 24.4g (0.0213mol) dissolved in 40ml of anhydrous toluene, POCl 3 30ml (0.3266mol), mix well, bl...
Embodiment 2
[0111] 6,7-Dimethoxy-1-(3,4-dimethoxy)benzyl-2-[N[2-(3',4'-dimethoxy)ethyl]glycyl] -1,2,3,4-tetrahydroisoquinoline (VI 2 ) preparation
[0112] V 1 Dissolve 3.8g (9.058mmol) in 50ml of dichloromethane, add 3,4-dimethoxyphenethylamine 3.28ml (18.1mmol), triethylamine 1.4ml (10.05mmol), catalytic amount of potassium iodide, and stir at room temperature for 14h . Chloroform:methanol=14:1 was used as the developer column chromatography to separate the main spot to obtain 2.0 g of oil. Yield 39.1%.
[0113] MS (ESI, m / z): 565 ([M+H] + , base peak)
[0114] 6,7-Dimethoxy-1-(3,4-dimethoxy)benzyl-2-[(chloroglycyl)-N-[2-(3',4'-dimethoxy ) ethyl] glycyl] -1,2,3,4-tetrahydroisoquinoline (VII 2 ) preparation
[0115] VI 2 5.7g (10.11mmol) was dissolved in 50ml of dichloromethane, DCC 2.3g (11.15mmol), DMAP catalytic amount was added, chloroacetic acid 1.92g (20.3mmol) was added in batches, room temperature was stirred for 1h, petroleum ether: ethyl acetate = 1:4 is the develope...
Embodiment 3
[0131] 6,7-dimethoxy-1-(3,4-dimethoxy)benzyl-2-(N-cyclohexylglycyl)-1,2,3,4-tetrahydroisoquinoline ( VI 4 ) preparation
[0132] V 1 Dissolve 3g (7.15mmol) in 30ml of dichloromethane, add 1.64ml (14.3mmol) of cyclohexylamine, 1.1ml (7.865mmol) of triethylamine, and a catalytic amount of potassium iodide, and stir at room temperature for 14h. Chloroform:methanol=14:1 was used as the developer column chromatography to separate the main spot to obtain 2.0 g of oil. Yield 58.0%.
[0133] MS (ESI, m / z): 483 ([M+H] + , base peak)
[0134] 6,7-Dimethoxy-1-(3,4-dimethoxy)benzyl-2-[(chloroglycyl)-N-cyclohexylglycyl]-1,2,3,4 -Tetrahydroisoquinoline (VII 4 ) preparation
[0135] VI 4 3.6g (7.47mmol) was dissolved in 30ml of dichloromethane, DCC 1.7g (8.22mmol), DMAP catalytic amount was added, chloroacetic acid 1.4g (14.9mmol) was added in batches, room temperature was stirred for 1h, petroleum ether: ethyl acetate = 1:4 is the developer column chromatography to separate the ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com