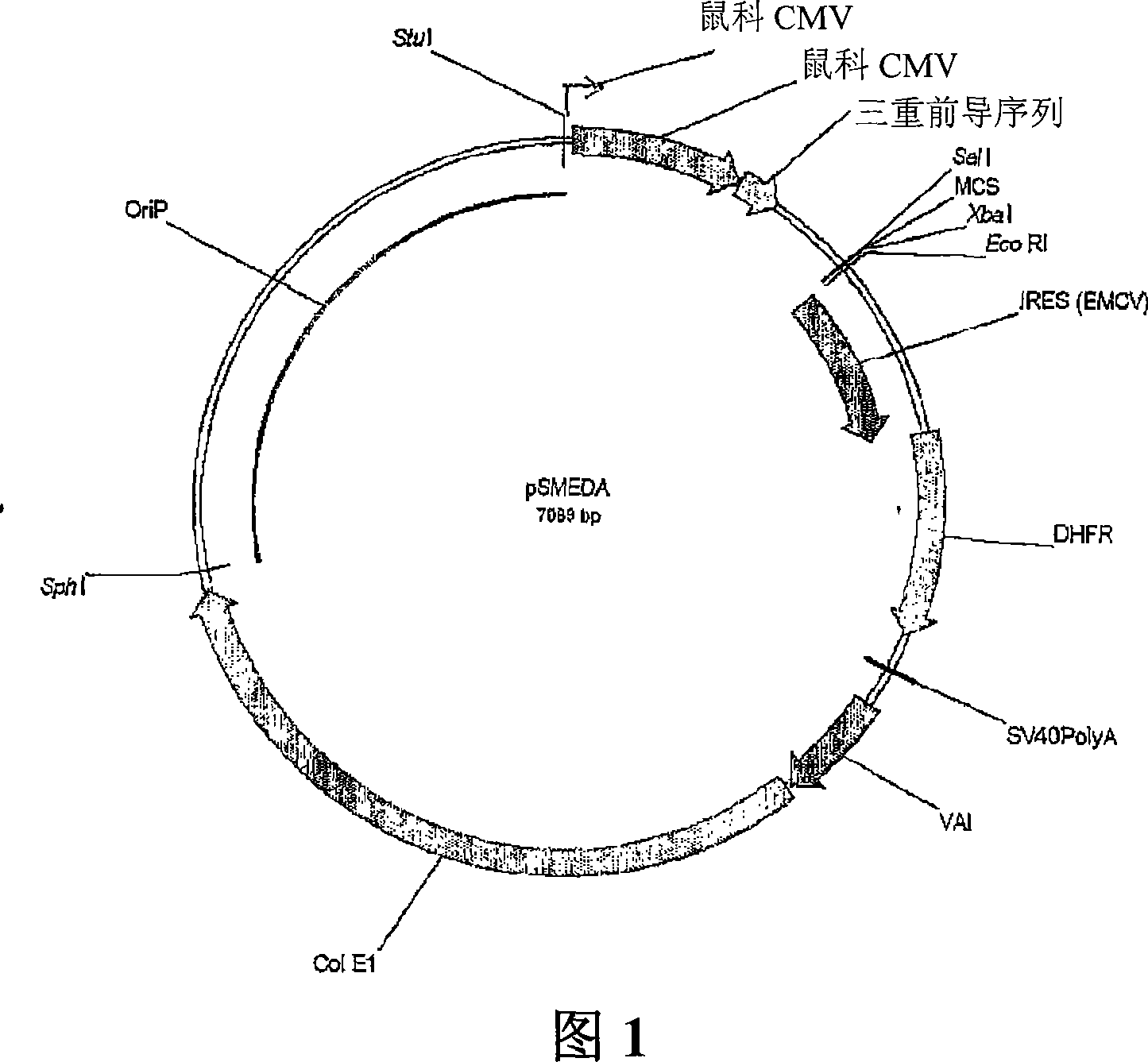
Mammalian expression systems
An expression system, mammalian technology, applied in the direction of digestive system, endocrine system diseases, animal/human proteins, etc., can solve the problem of not being able to produce a large amount of desired protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0091] Example 1. Cell Culture and DNA Constructs
[0092] Make mammalian cell lines (HEK293-FT, HEK293-EBNA, CHO-DUKX and Lec.3.2.8.1) in 5% CO 2 Grow and maintain at 37°C in a humidified incubator. HEK293 cells were cultured in dendritic 293 medium (Invitrogen) supplemented with 5% fetal bovine serum (FBS). The CHO-DUKX stable cell line was grown in alpha medium containing 10% FBS and 200 nM methotrexate (MTX). HEK293 stable cell lines were cultured in alpha medium containing 10% FBS and 100 nM MTX.
[0093] Perform transient expression in a 50 mL rotator or a 1 L rotator. For a 50-ml culture volume, 25 μg of plasmid DNA was mixed with 400 μg of polyethyleneimine (PEI, 25 kDa, linear, neutralized with HCl to pH 7.0, 1 mg / ml (Polysciences, Warrington, PA)) in 2.5 ml without Mix in serum-containing 293 medium. For 1 L culture volume, mix 500 µg DNA with 4 mg PEI in 50 mL serum-free 293 medium. The mixture was then mixed with 50 mL or 1 L of HEK293 cells at 0.5 x 10 6 Cell...
example 2
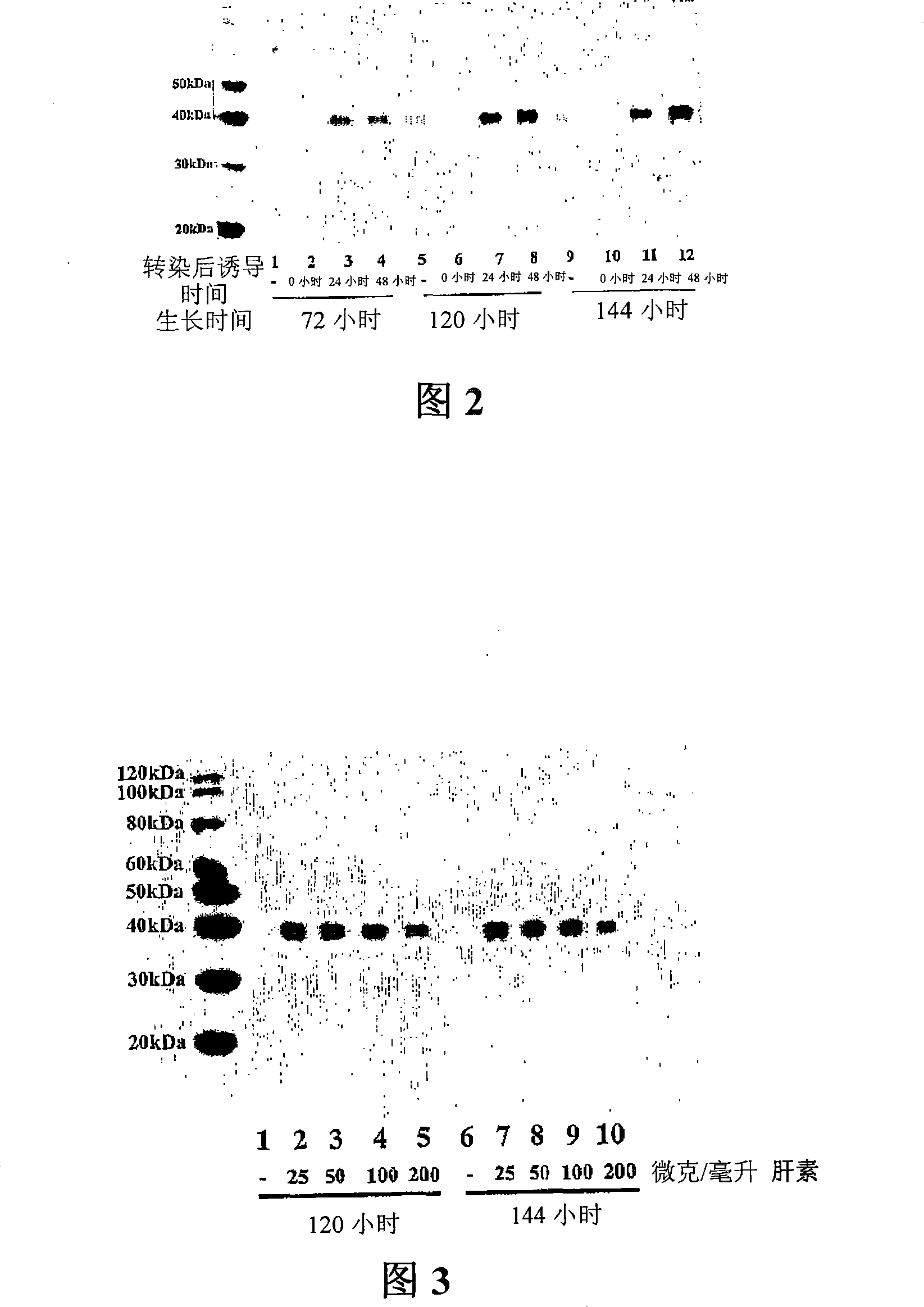
[0096] Example 2. Heparin enhances the production of sFRP-1 by transfected cells
[0097] The C-terminal his6-tagged sFRP-1 was transiently expressed in HEK293-FT cells as described in Example 1. 50 μg / ml heparin (Sigma Chemical) was added to the cell culture medium during or after DNA transfection ("post-transfection induction time", shown in Figure 2). Conditioned medium was harvested at different time points ("growth time", shown in Figure 2). Protein samples were separated by SDS-PAGE and subjected to Western blot analysis with mouse monoclonal anti-his4 antibody (Qiagen) at 0.2 μg / ml (Figure 2). Western blotting was performed according to Zhong et al. (2004) FEBS Lett.562: Implementation as described in 111-117. As shown in Figure 2, heparin greatly increased sFRP-1 production by transfected HEK293 cells. The greatest increase was observed when heparin was added to the medium 48 hours after DNA transfection. In other experiments, this increase was not observed with re...
example 3
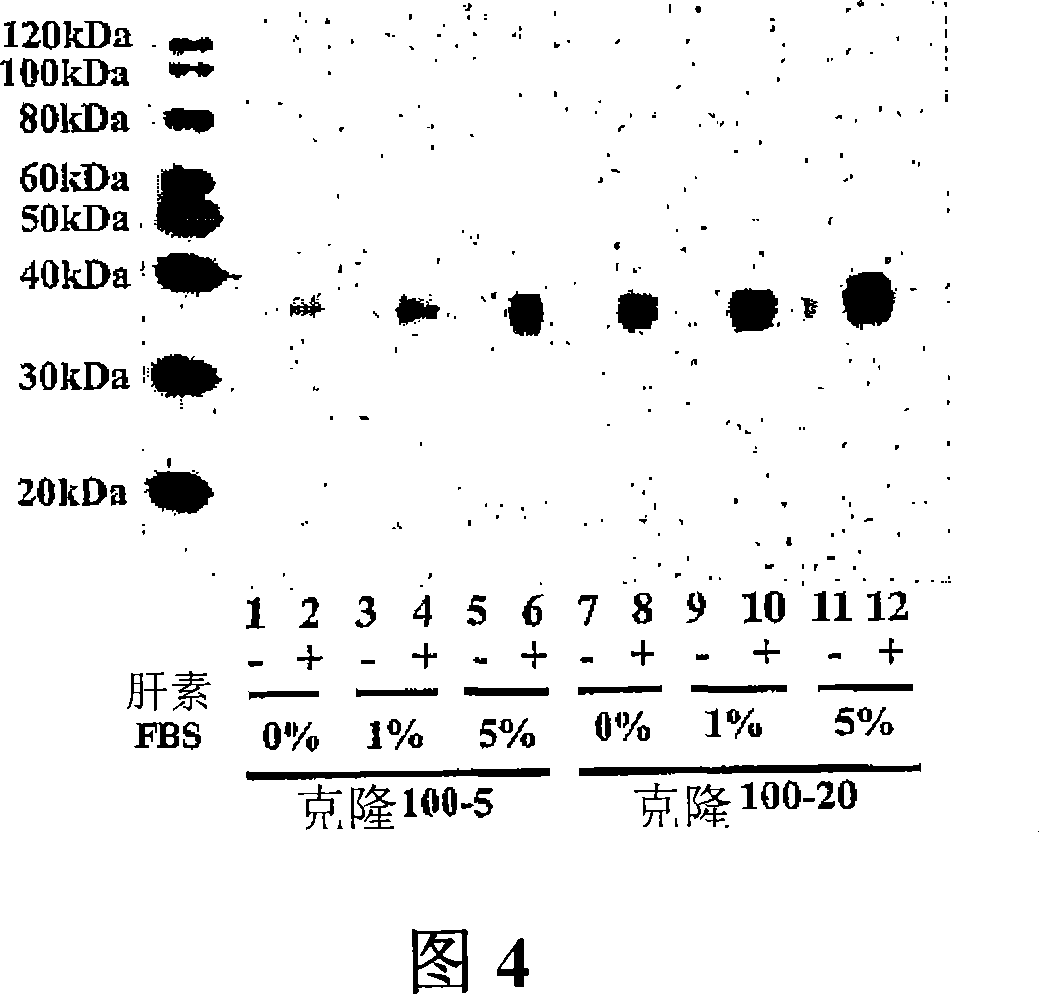
[0106] Example 3. FGF-2 Enhances Protein Production by Transfected Cells
[0107] Cells from the HEK293 cell line stably overexpressing sFRP-1 (clone 100-5) were grown to confluence in 6-well plates; the serum-free medium was then replaced with fresh serum-free medium containing 50 μg / ml heparin Medium. 50 ng / ml of fibroblast growth factor-1 (FGF-1, Sigma Chemical Company) or fibroblast growth factor-2 (FGF-2, Sigma Chemical Company) was added to the medium in the corresponding well. Conditioned medium was harvested 48 hours after medium replacement. Protein samples were separated by SDS-PAGE and subjected to immunoblot analysis with mouse monoclonal anti-his4 antibody (Figure 10). As demonstrated in Figure 10, the combination of FGF-2 and heparin significantly increased the production of sFRP-1 by stably transfected HEK293 cells.
[0108] Thus, FGF-2 and heparin can regulate protein synthesis in a post-transcriptional manner, as Northern blot analysis showed that sFRP-1 mR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com