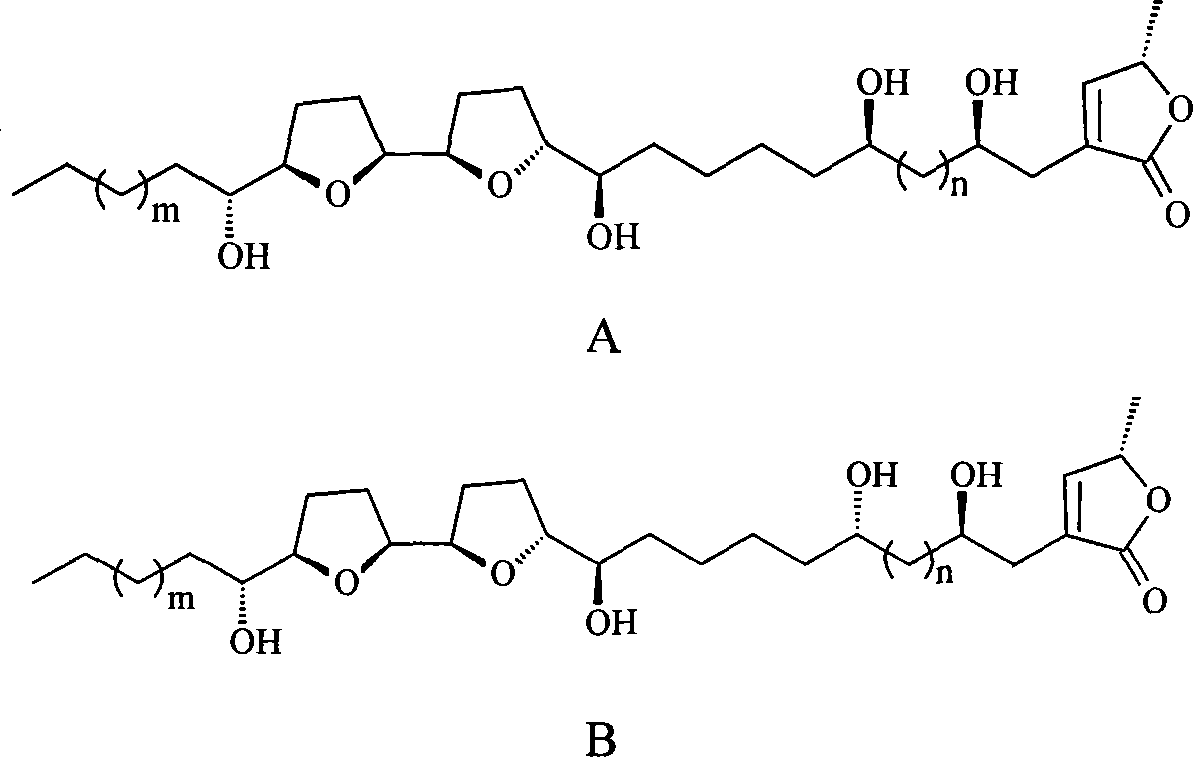
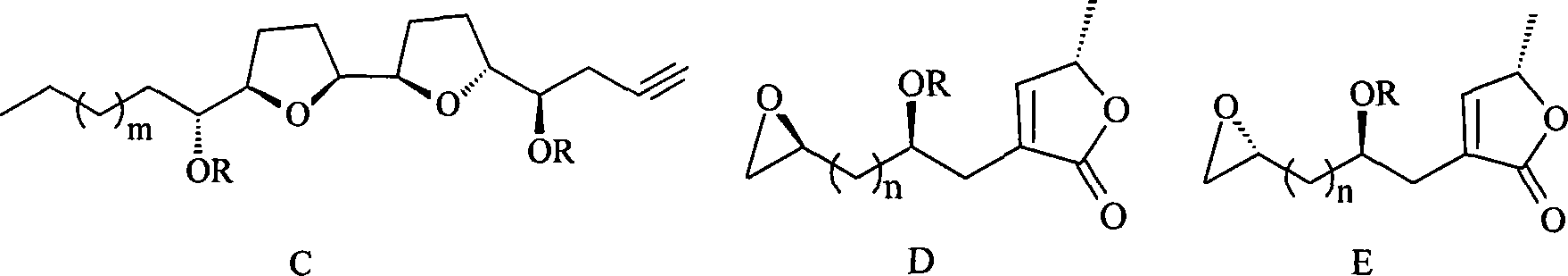
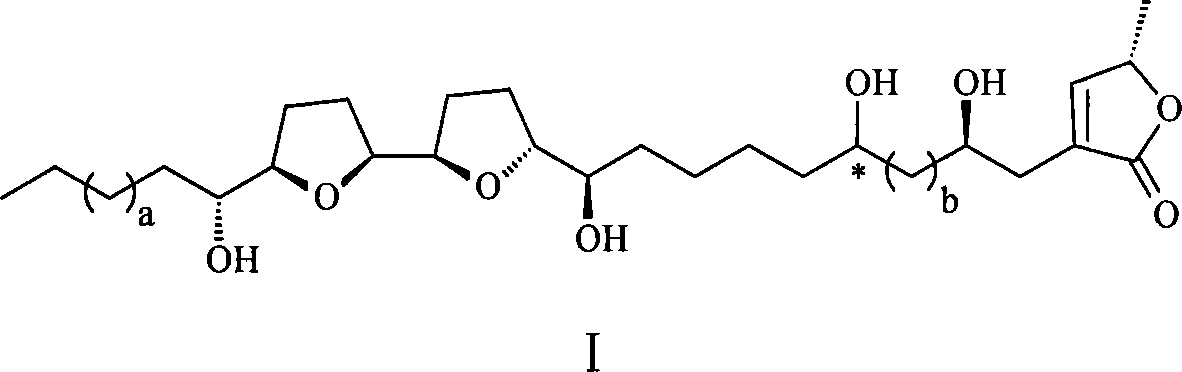
Annonaceousacetogenins compounds and preparation method thereof
A kind of technology of annua lactone and compound, which is applied in the field of tetrahydrofuran-containing annua lactone compound and its preparation, and can solve problems such as unsatisfactory performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023] Where: MOM is CH 2 OCH 3 (the same below)
[0024] Dissolve 0.32mmol of compound 4 in freshly distilled tetrahydrofuran solution, place it in a low-temperature tank at -78°C and stir for 10 to 15 minutes, then add 0.15mL of hexane solution with a concentration of 2.5Mn-BuLi dropwise, after the dropwise addition is complete, continue Stir for 30 minutes; then drop the tetrahydrofuran solution of compound 5 into the system, continue the reaction for 1 to 2 hours, quench the reaction with saturated ammonium chloride at 0°C, extract with ethyl acetate, and dry over anhydrous sodium sulfate , concentrated, and column chromatography gave 143 mg of the product (compound 3), with a yield of 55%.
[0025]
[0026] 0.29 mmol of compound 3 was dissolved in a mixture consisting of 0.5-10 ml of methanol, tetrahydrofuran and 6N hydrochloric acid, wherein the volume ratio of 6N hydrochloric acid, tetrahydrofuran and methanol was 1:1:2. Stir at room temperature for ab...
Embodiment 2
[0032] The structural formula is The ditetrahydrofuryne compound and the structural formula are The chiral epoxy compound reaction, operation is with embodiment 1, can obtain following object (compound 6):
[0033]
[0034] Structural characterization data of compound 6:
[0035] 1 HNMR (CDCl 3 , 400MHz) δ: 7.18(q, J=1.5Hz, 1H), 5.09(qq, J=6.8, 1.5Hz, 1H), 4.06(m, 1H), 3.97(m, 1H), 3.85(m, 1H ), 3.84(m, 2H), 3.59(m, 1H), 3.38(m, 2H), 2.53(m, 1H), 2.40(ddt, J=15.2, 8.5, 1.8Hz), 2.05(m, 1H) , 1.96(m, 2H), 1.70-1.45(m, 23H), 0.88(t, J=7.0Hz, 3H);
[0036] 13 CNMR (CDCl 3 , 100MHz): δ174.6, 151.9, 131.2, 83.5, 82.6, 81.5, 80.8, 78.2, 74.5, 73.6, 71.7, 70.0, 33.5, 28.8, 28.3, 19.0, 14.1.
Embodiment 3
[0038] The structural formula is The ditetrahydrofuryne compound and the structural formula are or The chiral epoxy compound reaction, operation is with implementing 1, can obtain following object (compound 7 and compound 8):
[0039]
[0040] Structural characterization data of compound 7:
[0041] 1 H-NMR (CDCl 3 , 400MHz) δ 7.18(q, J=1.5Hz, 1H), 5.09(qq, J=6.8, 1.5Hz, 1H), 4.06(m, 1H), 3.97(m, 1H), 3.85(m, 1H) , 3.84(m, 2H), 3.59(m, 1H), 3.38(m, 2H), 2.53(m, 1H), 2.40(ddt, J=15.2, 8.5, 1.8Hz), 2.05(m, 1H), 1.96(m, 2H), 1.70-1.45(m, 19H), 0.88(t, J=7.0Hz, 3H);
[0042] 13 CNMR (CDCl 3, 100MHz): δ174.6, 151.9, 131.2, 83.5, 82.6, 81.5, 80.8, 78.2, 74.5, 73.6, 71.7, 70.0, 43.6, 38.1, 33.5, 28.8, 28.3, 23.0, 19.0, 14.1.
[0043]
[0044] Structural characterization data of compound 8:
[0045] 1 H-NMR (CDCl 3 , 400MHz) δ 7.18(q, J=1.5Hz, 1H), 5.09(qq, J=6.8, 1.5Hz, 1H), 4.06(m, 1H), 3.97(m, 1H), 3.85(m, 1H) , 3.84(m, 2H), 3.59(m, 1H), 3.38(m, 2H), 2.53(m, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com