High-purity silybin meglumine, preparation method as well as application in preparing medicine treating hepatitis and liver hurt thereof
A technology of silibinin and meglumine, which is applied in the field of medicine, can solve the problems of low purity, achieve high purity, improve human bioavailability, eliminate potential safety hazards in clinical application and poor quality controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
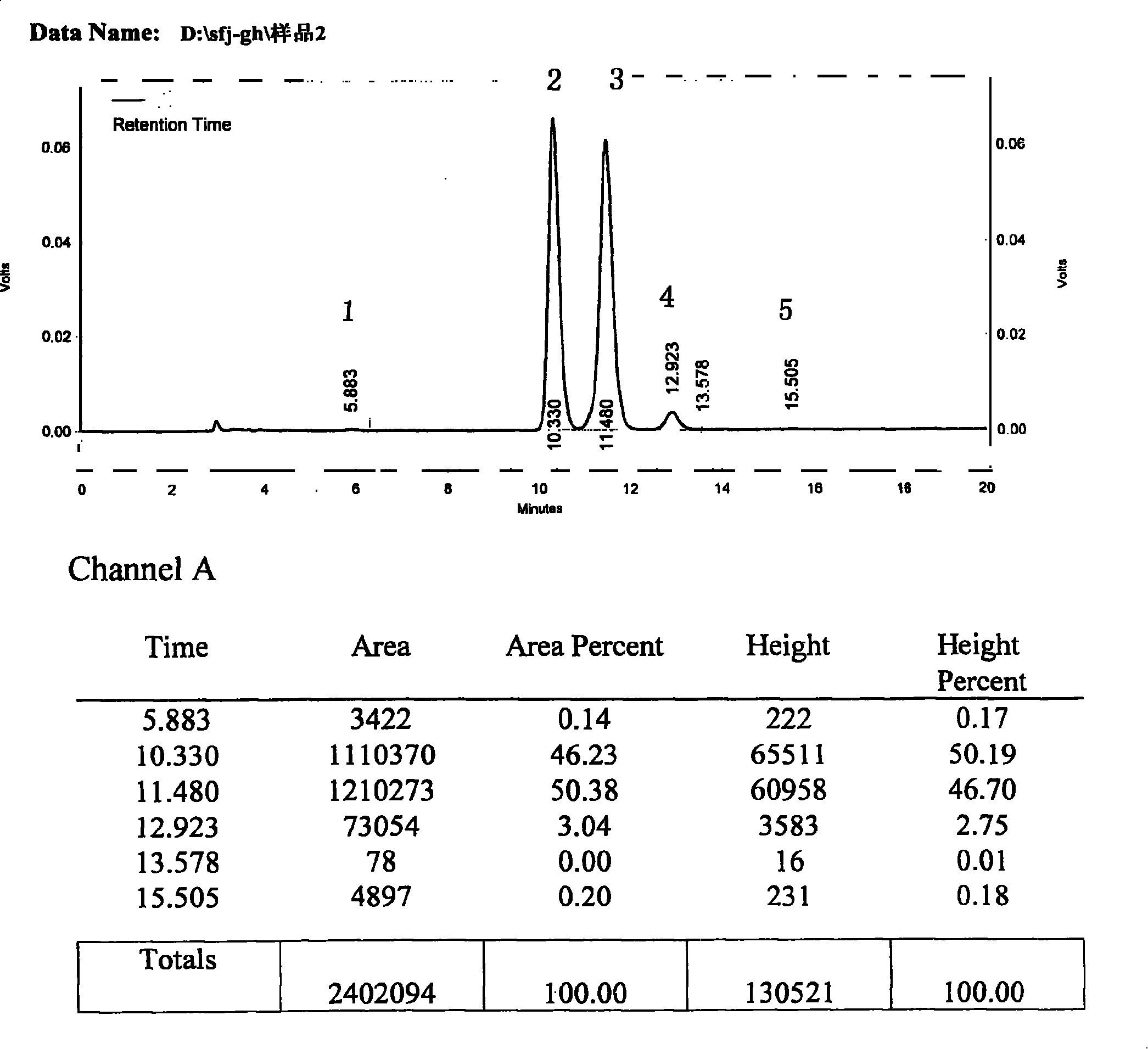
[0134] Take 30g of commercially available silybin meglumine, add 1500ml of isopropanol, heat and reflux to dissolve the sample, keep it for 0.5 hours, filter while it is hot, place the filtrate in a refrigerator at 4°C for 8 hours, filter, and concentrate the filtrate at 70°C under reduced pressure Evaporate the solvent to dryness, and dry under vacuum at 40°C for 24 hours to obtain a khaki powder; add 360ml of distilled water, stir at 10°C for 10 minutes to dissolve, filter, add 480ml of saturated saline to the filtrate, place it in a refrigerator at 4°C overnight, filter, and filter at 40°C Vacuum dry for 24 hours to obtain a light yellow powder; add 2000ml of isopropanol, heat to reflux to dissolve the sample, filter while it is hot, place the filtrate in a refrigerator at 4°C for 8 hours, filter, and concentrate the filtrate at 70°C under reduced pressure to evaporate the solvent to dryness, 40 °C and vacuum-dried for 24 hours to obtain 16.8 g of light yellow powder.
[01...
Embodiment 2
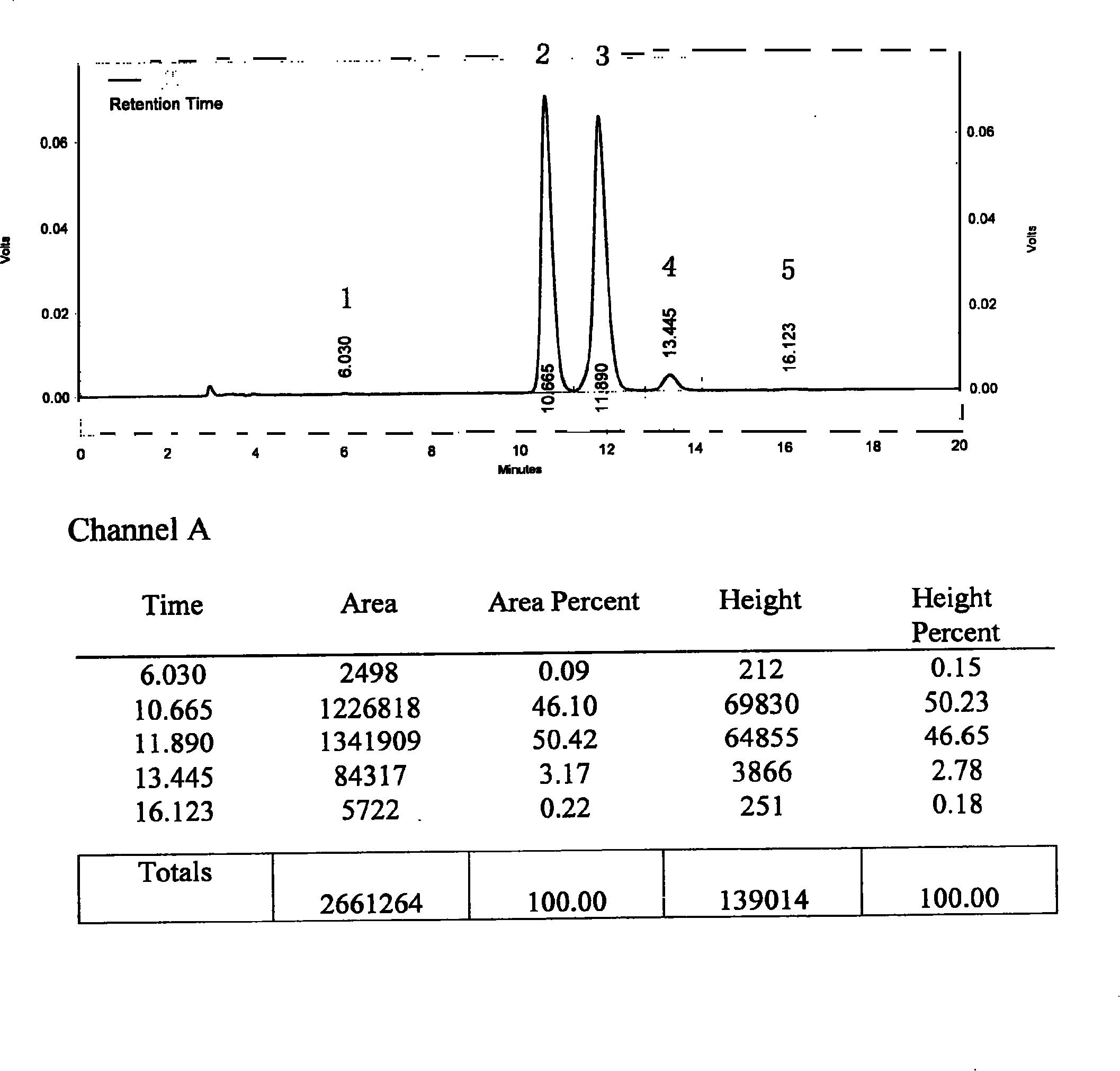
[0140] Take 30g of commercially available silybin meglumine, add 900ml of isopropanol, heat and reflux to dissolve the sample, keep it for 45 minutes, filter while it is hot, place the filtrate in a refrigerator at 4°C for 8 hours, filter, and concentrate the filtrate under reduced pressure at 40°C Evaporate the solvent to dryness, and dry in vacuum at 40°C for 24 hours to obtain a khaki powder; add 120ml of distilled water, stir at 0°C for 10 minutes to dissolve, filter, add 240ml of saturated saline to the filtrate, place it in a refrigerator at 4°C overnight, filter, and filter at 40°C Vacuum dry for 24 hours to obtain a light yellow powder; add 1200ml of isopropanol, heat to reflux to dissolve the sample, filter while it is hot, place the filtrate in a refrigerator at 4°C for 8 hours, filter, and concentrate the filtrate at 40°C under reduced pressure to evaporate the solvent to dryness, 40 °C and vacuum dried for 24 hours to obtain 17.5 g of light yellow powder.
[0141] ...
Embodiment 3
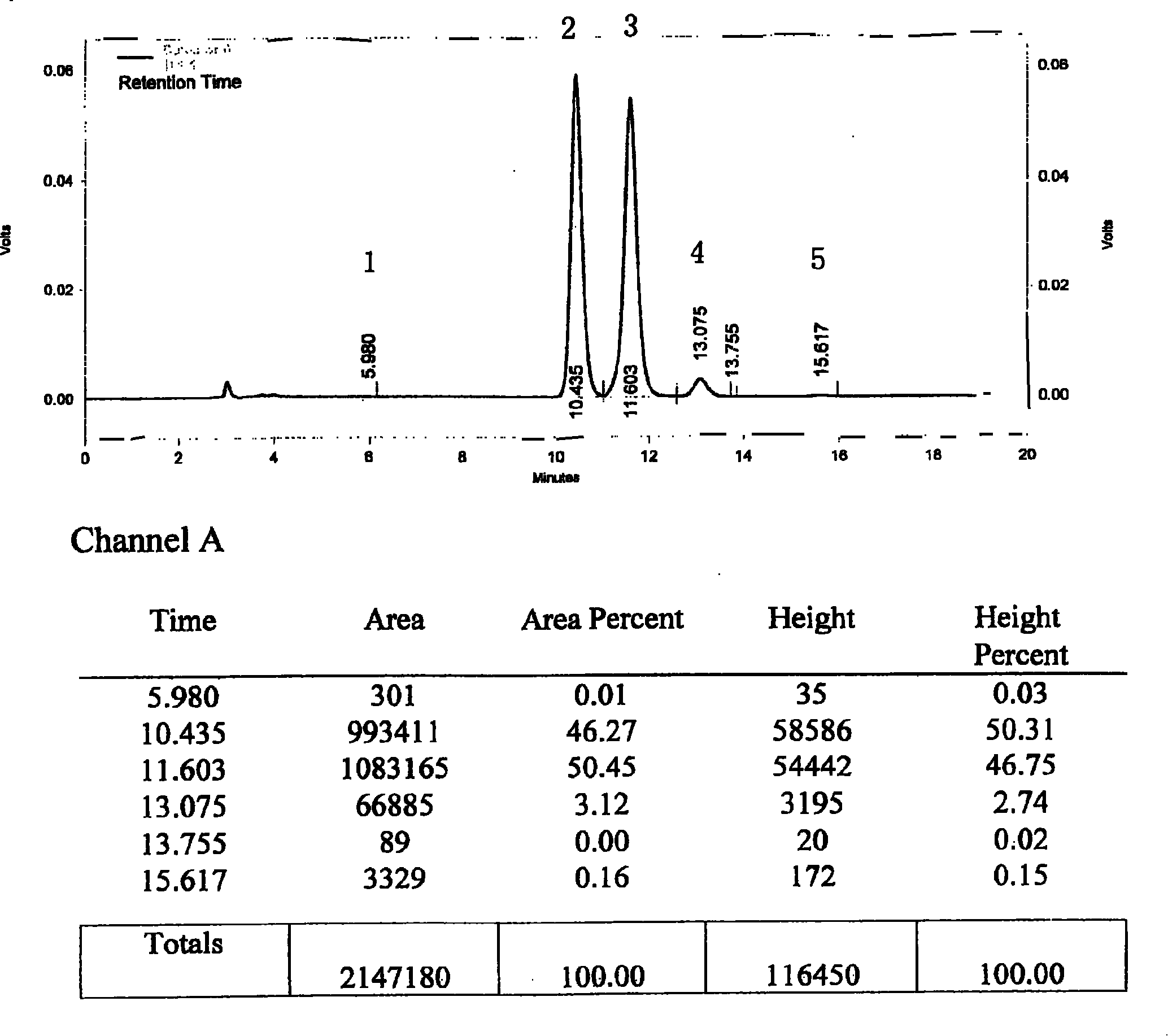
[0143] Take 30g of commercially available silybin meglumine, add 1200ml of isopropanol, heat and reflux to dissolve the sample, keep it for 15 minutes, filter while it is hot, place the filtrate in a refrigerator at 4°C for 8 hours, filter, and concentrate the filtrate at 60°C under reduced pressure Evaporate the solvent to dryness, and dry under vacuum at 40°C for 24 hours to obtain a khaki powder; add 240ml of distilled water, stir at 3°C for 10 minutes, dissolve, filter, add 360ml of saturated saline to the filtrate, place it in a refrigerator at 4°C overnight, filter, and filter at 40°C Vacuum dry for 24 hours to obtain a light yellow powder; then add 1600ml of isopropanol, heat to reflux to dissolve the sample, filter while it is hot, place the filtrate in a refrigerator at 4°C for 8 hours, filter, and concentrate the filtrate at 50°C under reduced pressure to evaporate the solvent to dryness, 40 °C and vacuum dried for 24 hours to obtain 18.5 g of light yellow powder. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com