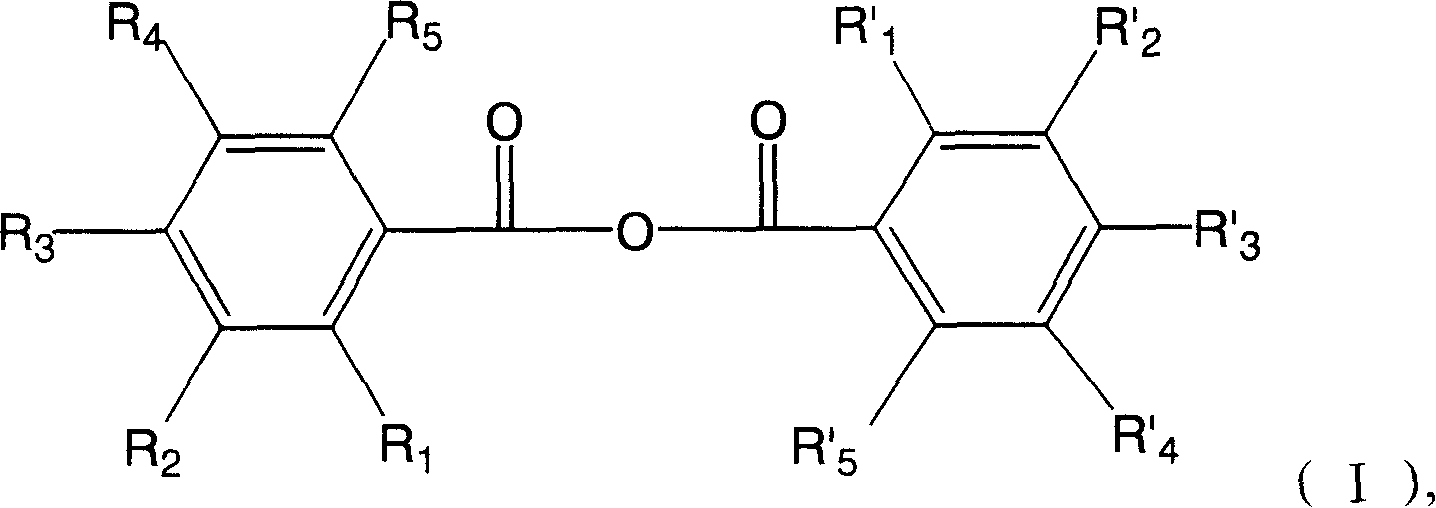
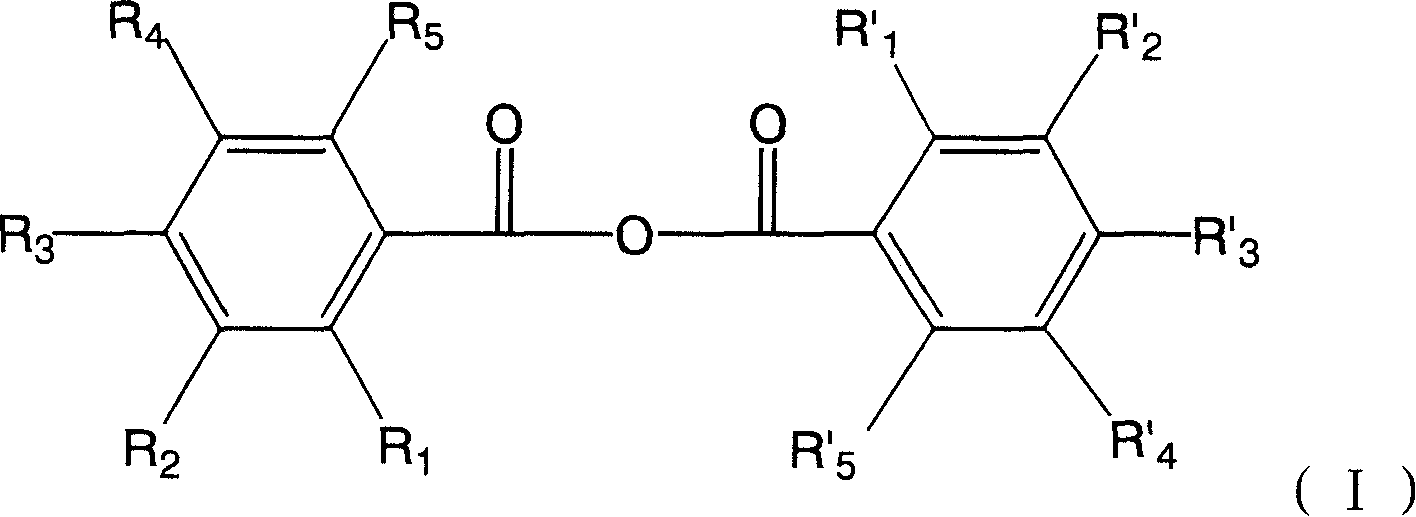
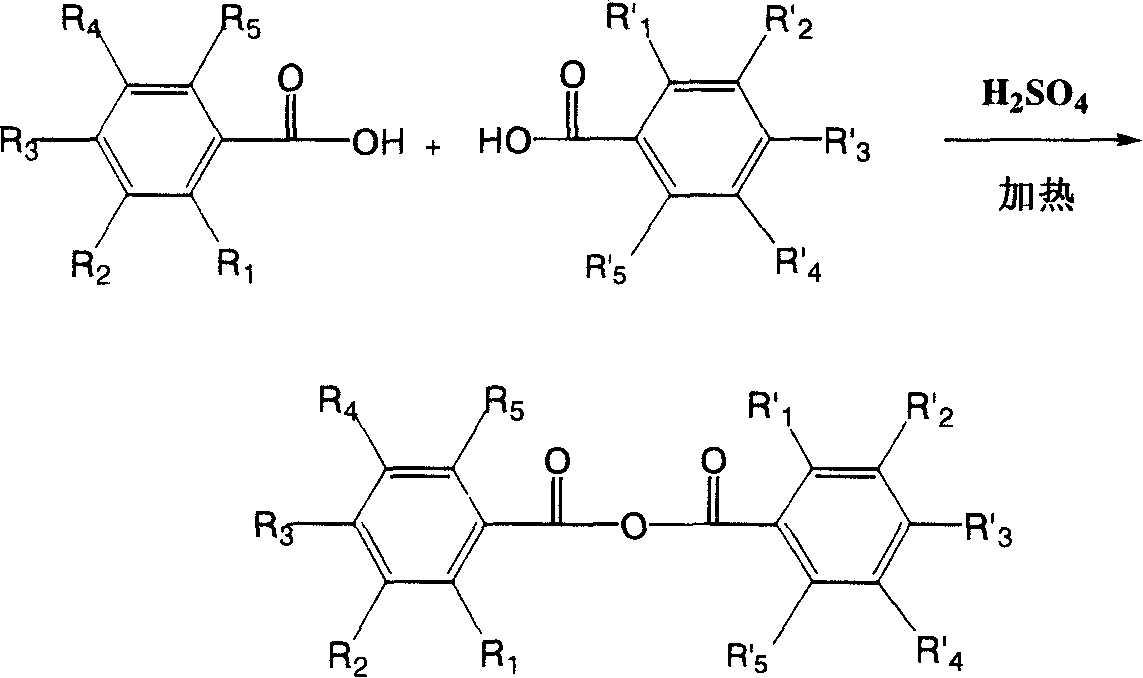
Hydroxyl benzoic anhydride dimer compound, preparation method and uses thereof
A technology of hydroxybenzoic anhydride dimer and tetrahydroxydibenzoic anhydride is applied in the fields of hydroxybenzoic anhydride dimer compound, preparation and use, and can solve problems such as low activity and instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 3,3',4,4',5,5'-hexahydroxydibenzoic anhydride
[0045] Get a 200 milliliter beaker, dissolve 20 grams of 3,4,5-trihydroxybenzoic acid in 100 grams of 1,4-dioxane, add 1 gram of concentrated sulfuric acid (5%) in the solution, and heat it in a Sanyo microwave oven 2450 ( MHz) to dissolve completely, then heated for 3 minutes (200°C), then evaporated 1,4-dioxane to dryness, recrystallized with acetone, then filtered, and dried at 70°C to obtain a reddish-orange substance , yield 96%.
[0046] 1 HNMR (400MHz, DMSO): δ6.901(6H), δ2.486-2.494(4H).IR(KBr, CM -1 ): 3463.6, 3059.2, 2667.5, 1917.4, 1867.5, 1828.9, 1794.6, 1657.6, 1614.7, 1542.6, 1454.5, 1434.8, 1318.0, 1265.5, 1227.4, 1170.1-471.2.
Embodiment 2
[0048] 3,3',4,4'-tetrahydroxydibenzoic anhydride
[0049] Get a 200 ml beaker, dissolve 20 grams of 3,4-dihydroxybenzoic acid in 100 grams of toluene, add 0.5 grams of p-toluenesulfonic acid in the solution, and heat it in a Sanyo microwave oven at 2450 (MHz) until it is completely dissolved, and then heat for 5 Minutes (150°C), then, evaporate toluene to dryness, recrystallize with acetone, filter, and dry at 70°C to obtain an orange substance, 3,3',4,4'-tetrahydroxydibenzoic anhydride, Yield 86%.
[0050] 1 HNMR (400MHz, DMSO): δ6.756-7.321 (4H), δ2.485-2.494 (2H).IR (KBr, CM -1 ): 3546.2, 3205.5, 2652.6, 1871.2, 1845.1, 1676.3, 1600.8, 1526.6, 1466.8, 1419.6, 1380.0, 1346.1, 1298.24, 1190.6, 1126.9-450.9.
Embodiment 3
[0052] 2,2',3,3'-tetrahydroxydibenzoic anhydride
[0053] Take a 200 ml beaker, dissolve 20 grams of 2,3-dihydroxybenzoic acid in 100 grams of benzene, add 2 grams of phosphoric acid and 2 grams of acetic anhydride in the solution, heat it in a Sanyo microwave oven at 2450 (MHz) until completely dissolved, and then Heated for 6 minutes (100°C), then evaporated benzene to dryness, recrystallized with acetone, filtered, and dried at 70°C to obtain an orange substance, 2,2',3,3'-tetrahydroxybenzidine Anhydride, yield 82%.
[0054] 1 HNMR (400MHz, DMSO): δ6.99-7.24(4H), δ2.49(2H).IR(KBr, CM -1 ): IR (KBr, CM -1 ):3377.2,3243.0,3096.5,2578.6,1916.3,1847.1,1794.7,1678.8,1656.6,1598.9,1559.8,1540.9,1474.7,1433.7,1382.37,1354.0,1301.7,1258.0,1233.46,1158.65,1070.3-419.3
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com