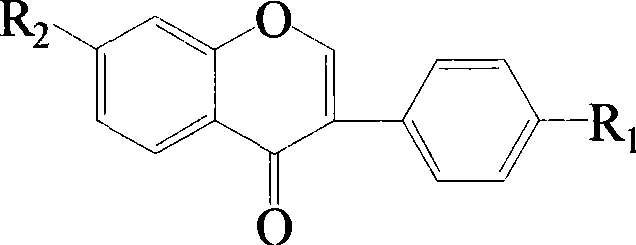
Isoflavone sulfonate derivatives and synthetic method thereof
A technology of isoflavone sulfonate and synthesis method, which can be applied in the directions of drug combination, pharmaceutical formula, organic active ingredient, etc., can solve the problems of poor absorption, slow effect and low activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
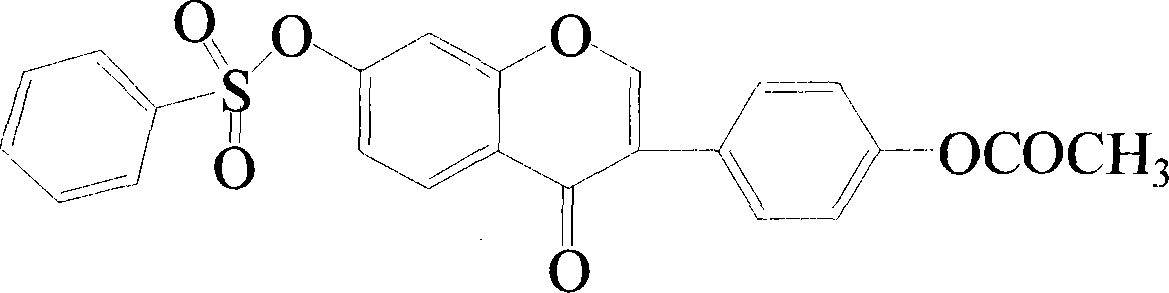
[0018] Example 1 Synthesis of Compound 4'-Acetoxyl-7-Hydroxy Isoflavones
[0019] Synthesis of intermediate 4'-hydroxy-7-benzenesulfonyloxyisoflavone. Take daidzein (0.4mmol, 0.1016g) and disperse it in 10mL of dichloromethane, add 0.01g of potassium tert-butoxide, and slowly add 0.48mmol (0.0847g) of benzenesulfonyl chloride dropwise at -20°C under the protection of Ar . Incubate for 0.5-24 hours. Filter the mixture, spin the filtrate to dryness under reduced pressure, and purify by column chromatography (chloroform / acetone=10:1) to obtain a pure product.
[0020] 1 H NMR (400MHz, CDCl 3 ): δ 8.21 (1H, d, J = 8.8Hz), 7.97 (1H, s), 7.90 (2H, d, J = 7.6Hz,), 7.72 (1H, t, J = 8.0Hz,), 7.58 ( 2H, t, J=7.6, 8.0Hz,), 7.43 (2H, d, J=8.4Hz,), 6.98 (1H, d, 4 J = 2.0Hz), 6.90 (2H, d, J = 8.4Hz,), 6.86 (1H, dd, J = 2.0, 8.8Hz), , 5.00 (1H, s); m / z (EI) 395.12 (M + +1, 100%).
[0021] Synthesis of 4'-Acetoxy-7-Hydroxyisoflavones. Take the intermediate 4'-hydroxyl-7-benzenesulfony...
Embodiment 2
[0023] Example 2 Synthesis of compound 4'-methoxy-7-benzenesulfonyloxyisoflavone Method 1: Synthesis of 4'-methoxy-7-benzenesulfonyloxyisoflavone. Take 0.4mmol intermediate 4'-hydroxyl-7-benzenesulfonyloxy isoflavone 6 (0.1576g) and dissolve it in 10mL acetone, add 0.1g potassium carbonate, add 0.15mL dimethyl sulfate at room temperature, at 25 ℃~60℃ for 5 hours. The reaction mixture was filtered, the filtrate was spin-dried, and purified by column chromatography (chloroform / acetone=5:1) to obtain a pure product. Method 2: Synthesis of intermediate 4'-methoxy-7-hydroxyisoflavone. Take daidzein (0.4mmol, 0.1016g) and disperse it in 10mL of dimethylformamide, add 0.1g of potassium carbonate, add 0.15mL of dimethyl sulfate at room temperature, and react at 25°C to 60°C for 3h. The reaction solution was added to 20 mL of cold water, filtered, the filter cake was washed with water, and purified by column chromatography to collect the second band. White solid, 0.040g, yield 25%, ...
Embodiment 3
[0025] Example 3 Synthesis of Compound 4'-Ethoxy-7-Benzenesulfonyloxy Isoflavone
[0026] Method 1: Synthesis of 4'-ethoxy-7-benzenesulfonyloxy isoflavone, take 0.4mmol compound 6 (0.1576g) and dissolve it in 10mL acetone, add 0.1g potassium hydroxide and 0.2L water, in Slowly add 0.15mL diethyl sulfate at room temperature, and react at 25°C~60°C for 5h. The reaction mixture was filtered, the filtrate was spin-dried, and purified by column chromatography (chloroform / acetone=5:1) to obtain a pure product.
[0027] Method 2: Synthesis of intermediate 4'-ethoxy-7-hydroxyisoflavone. Take daidzein (0.4mmol, 0.1016g) and disperse it in 10mL dimethylformamide, add 0.1g potassium carbonate, 0.15 mL of diethyl sulfate was added under low temperature, and the reaction was carried out at 25° C. to 60° C. for 3 h. The reaction solution was added to 20 mL of cold water, filtered, the filter cake was washed with water, and purified by column chromatography to collect the first band. Whit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com