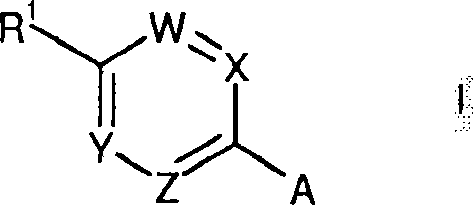
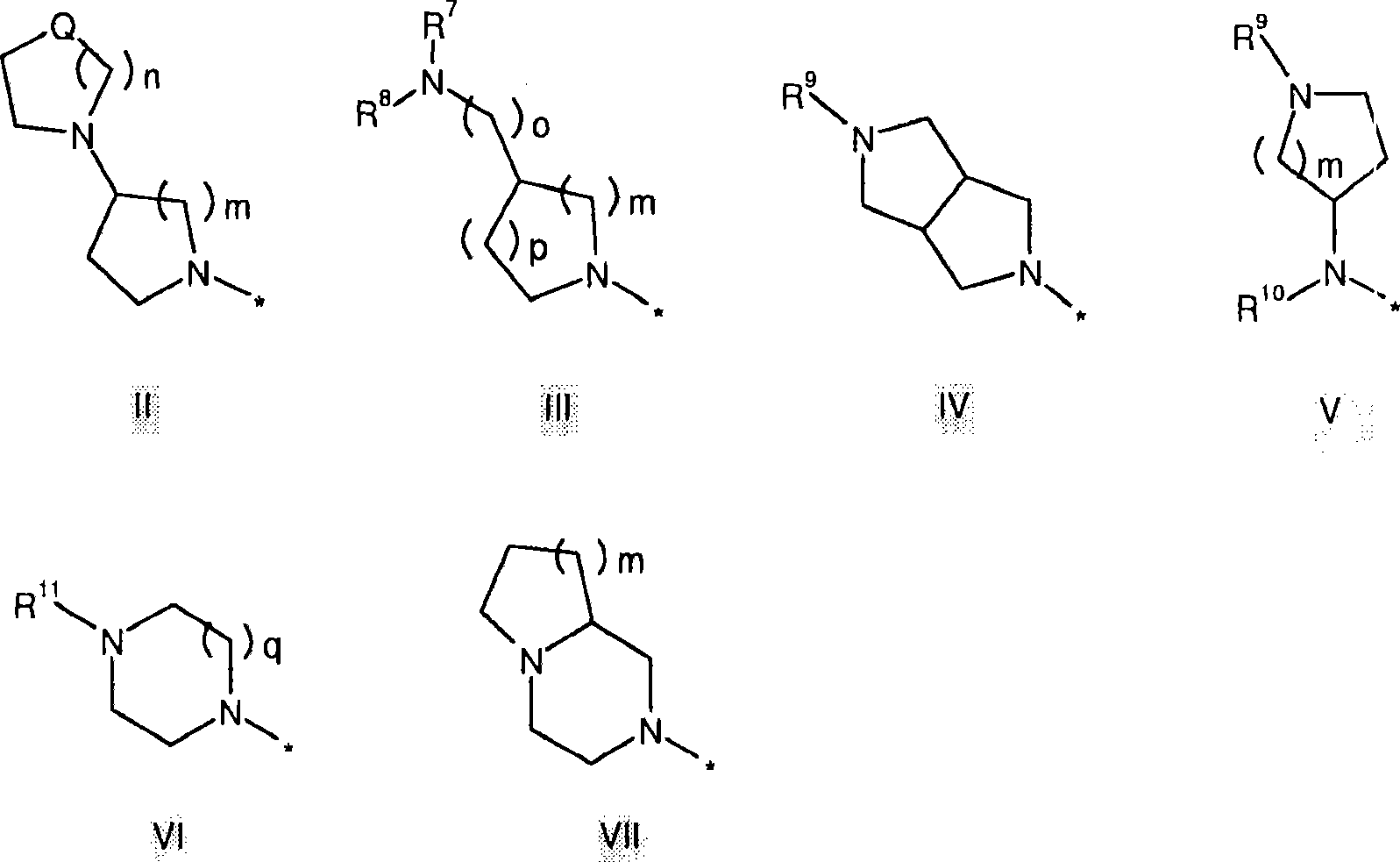
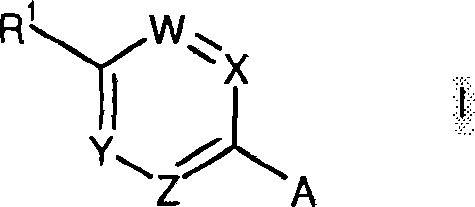
Histamine H3 receptor antagonists
An alkyl, general formula technology, applied in the field of new compounds, can solve the problem of not giving the pharmacological data of the compound and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0440] Example 1 (General Method A)
[0441] 1-[5-(4-Chlorophenyl)pyridin-2-yl]-4-isopropylpiperazine, dihydrochloride
[0442]
[0443]2-Chloro-5-(4-chlorophenyl)pyridine (500 mg, 2.23 mmol), DMSO (2.0 mL) and 1-isopropylpiperazine (3 mL, 23.4 mmol) were stirred and heated in a 100 °C oil bath overnight. The reaction mixture was poured into water (75 mL), and the solid was isolated by filtration, washed with water and dried. The crude product was purified by column chromatography on silica gel (Kiselgel 60, 0.040-0.63 mesh), washing with a mixture of ethyl acetate and methanol (4:1). Collection of appropriate fractions afforded 600 mg (85%) of 1-[5-(4-chlorophenyl)-pyridin-2-yl]-4-isopropylpiperazine.
[0444] 1 H NMR (300MHz, CDCl 3 )δ1.10 (d, 6H), 2.63-2.68 (m, 4H), 2.75 (septet, 1H), 3.59-3.66 (m, 4H), 6.69 (d, 1H), 7.35-7.45 (m, 4H ), 7.67 (dd, 1H), 8.40 (d, 1H).
[0445] The free base was dissolved in a mixture of 0.5N hydrochloric acid solution and ethanol. A...
Embodiment 2
[0450] Embodiment 2 (general method A)
[0451] 1-Isopropyl-4-[5-(4-methoxyphenyl)pyridin-2-yl]piperazine, dihydrochloride
[0452]
[0453] 2-Chloro-5-(4-methoxyphenyl)pyridine (200 mg, 0.91 mmol), anhydrous DMSO (2.0 mL) and 1-isopropylpiperazine (500 mg, 3.9 mmol) were stirred and heated at 130° C. Heat in an oil bath overnight, then heat at 150°C for 4 hours, leave at room temperature for 2 days, and finally heat at 140°C overnight. The reaction mixture was cooled, then poured into water (100 mL). The mixture was extracted with ethyl acetate (100 mL), and the organic extract was washed with water and dried (MgSO 4 ). The solvent was evaporated to give a solid residue which was dissolved in 0.5N hydrochloric acid (20 mL). A small amount of insoluble solid was removed by filtration and the aqueous solution was evaporated to give a residue which was re-evaporated with absolute ethanol. The resulting solid was recrystallized from absolute ethanol to afford 210 mg (60%)...
Embodiment 3
[0458] Embodiment 3 (general method A)
[0459] 1-Isopropyl-4-[5-(4-trifluoromethoxyphenyl)pyridin-2-yl]piperazine, dihydrochloride
[0460]
[0461] Starting from 2-chloro-5-(4-trifluoromethoxyphenyl)pyridine and 1-isopropylpiperazine, in a similar manner to that described in Example 1, the title compound was prepared.
[0462] Mp=271-273°C.
[0463] 1 H NMR (300MHz, DMSO-d 6 )δ1.32(d, 6H), 3.05-3.21(m, 2H), 3.45-3.67(m, 5H), 4.50-4.61(m, 2H), 7.28(d, 1H), 7.46(d, 2H) , 7.82 (d, 2H), 8.17 (d, 1H), 8.46 (s, 1H), 11.4 (brs, 1H).
[0464] C 19 h 22 f 3 N 3 Microanalysis of O, 2 x HCl:
[0465] Calculated value: C: 52.06%; H: 5.52%; N: 9.59%;
[0466] Measured values: C: 52.07%; H: 5.53%, N: 9.36%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com