Application of colon bacillus OmpA as animal immunity regulator
A technology of Escherichia coli and animal immunization, which is applied in the direction of medical preparations containing active ingredients, drug combinations, and resistance to vector-borne diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Immunomodulatory effect of Escherichia coli OmpA gene deletion strain
[0015] 1. Bacterial culture: Escherichia coli OmpA gene deletion strain was obtained from Nara Institute of Science and Technology, Japan. Pick a single colony and culture it overnight in LB medium, then inoculate it in LB medium at a ratio of 1:100, culture to OD1.0; centrifuge to obtain bacteria. At the same time, the source strain of Escherichia coli OmpA gene deletion strain was used as the control strain.
[0016] 2. Immunization of mice: domesticate the bought mice for 1 week, and feed artificial compound feed twice a day in the morning and evening. The mice with no abnormality after 1 week of domestication were divided into groups. Take the purified bacteria prepared above as immunogen, and immunize mice by intraperitoneal injection, 10 9 / Only. Three injections were given, the second injection was given 14 days after the first injection, and the third injection was given 7 days later. B...
Embodiment 2
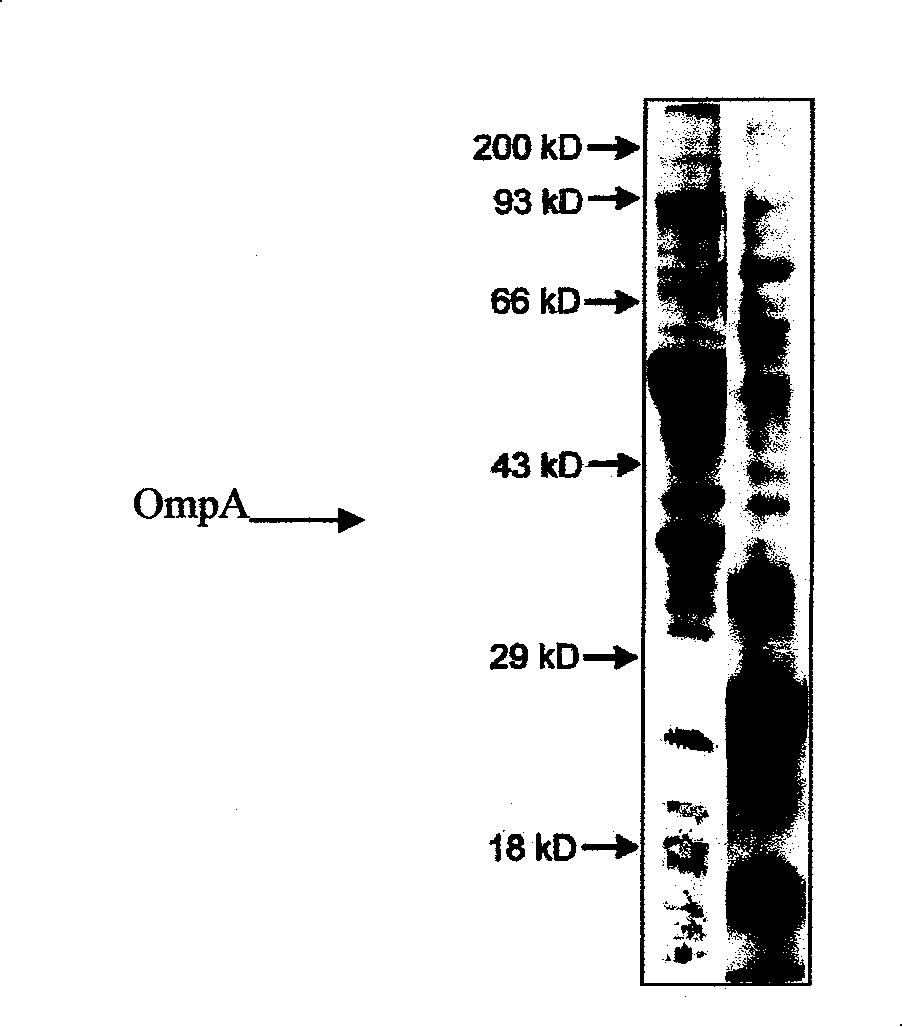
[0018] Preparation and purification of Escherichia coli OmpA
[0019] In this example, the full-length Escherichia coli OmpA coding sequence or fragments were constructed into the E. coli protein prokaryotic expression vector to express and purify the target protein.
[0020] The coding sequence of OmpA gene is as follows:
[0021] 1 ttaagcctgc ggctgagtta caacgtcttt gataccttta acttcgatct ctacgcgacg
[0022] 61 atccggagcc aggcagtcga tcagtgcagc acgctgtttc acgttgtcac aggtgttgcc
[0023] 121 agtaaccggg ttggattcgc ccataccacg tgcggagatc ttgtctgccg ggataccttt
[0024] 181 ggagatcagg taatcaacaa cagactgagc acggcgctcg gacagaccct ggttgtaagc
[0025] 241 gtcagaaccg atgcggtcgg tgtaacccag aacaactacg gaaccgtctt tcggatccag
[0026] 301 gttgctcagc tggctgtaca gctgatccag agcagcctga ccttccggtt tcagggttgc
[0027] 361 tttgttgaag ttgaacagaa cgtcagactt cagagtgaag tgcttggtct gtacttccgg
[0028] 421 tgccggagct ggagccggag caactactgg agctgcttcg ccctgaccga aacggtagga
[0029] 481 aacac...
Embodiment 3
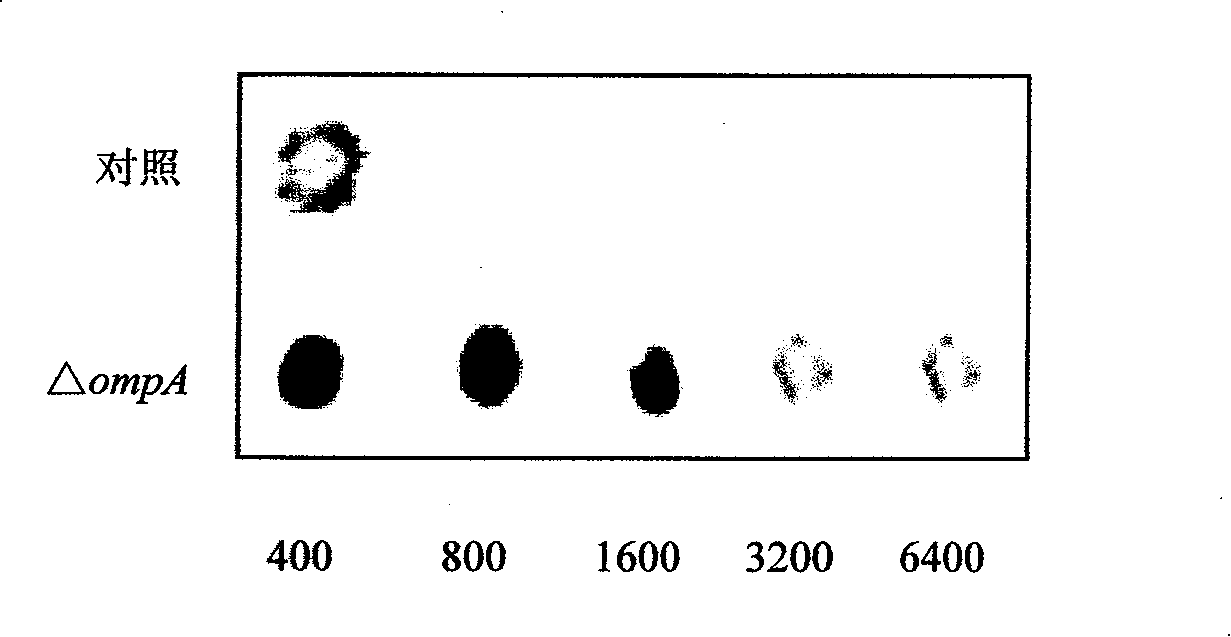
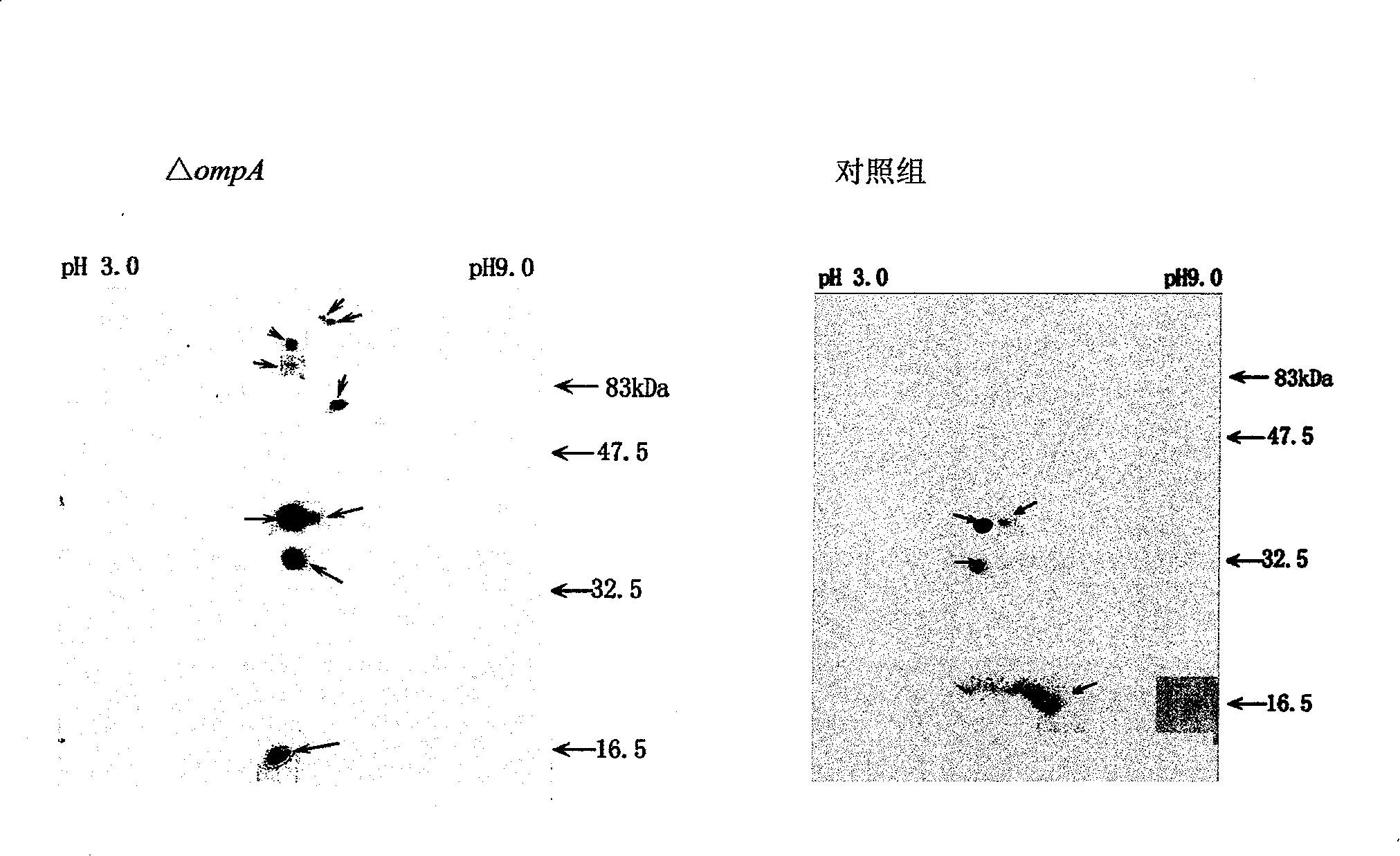
[0054] The immune enhancement effect of E. coli OmpA:
[0055] 1. Mice immunization: domesticate the bought mice for 1 week, and feed artificial compound feed twice a day in the morning and evening. The mice with no abnormality after 1 week of domestication were divided into groups. Take the purified OmpA and mix it with an equal volume of complete adjuvant (lanolin: paraffin oil = 1:4v / v, BCG: 30mg / ml), and after repeated suction with a syringe, ultrasonic treatment for 2min, the prepared antigen is injected intraperitoneally Immune mice, 80-100μg / only, the control group does not receive immune treatment. Inject 3 times with an interval of 10 days. The second and third times in the experimental group were mixed with incomplete adjuvant and purified protein before immunization. Blood was collected on the fifth day after the third immunization, and the serum immune titer was detected by Dot-ELISA method.
[0056] 2. Determination of serum titer: Dot-ELISA technology was used...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com