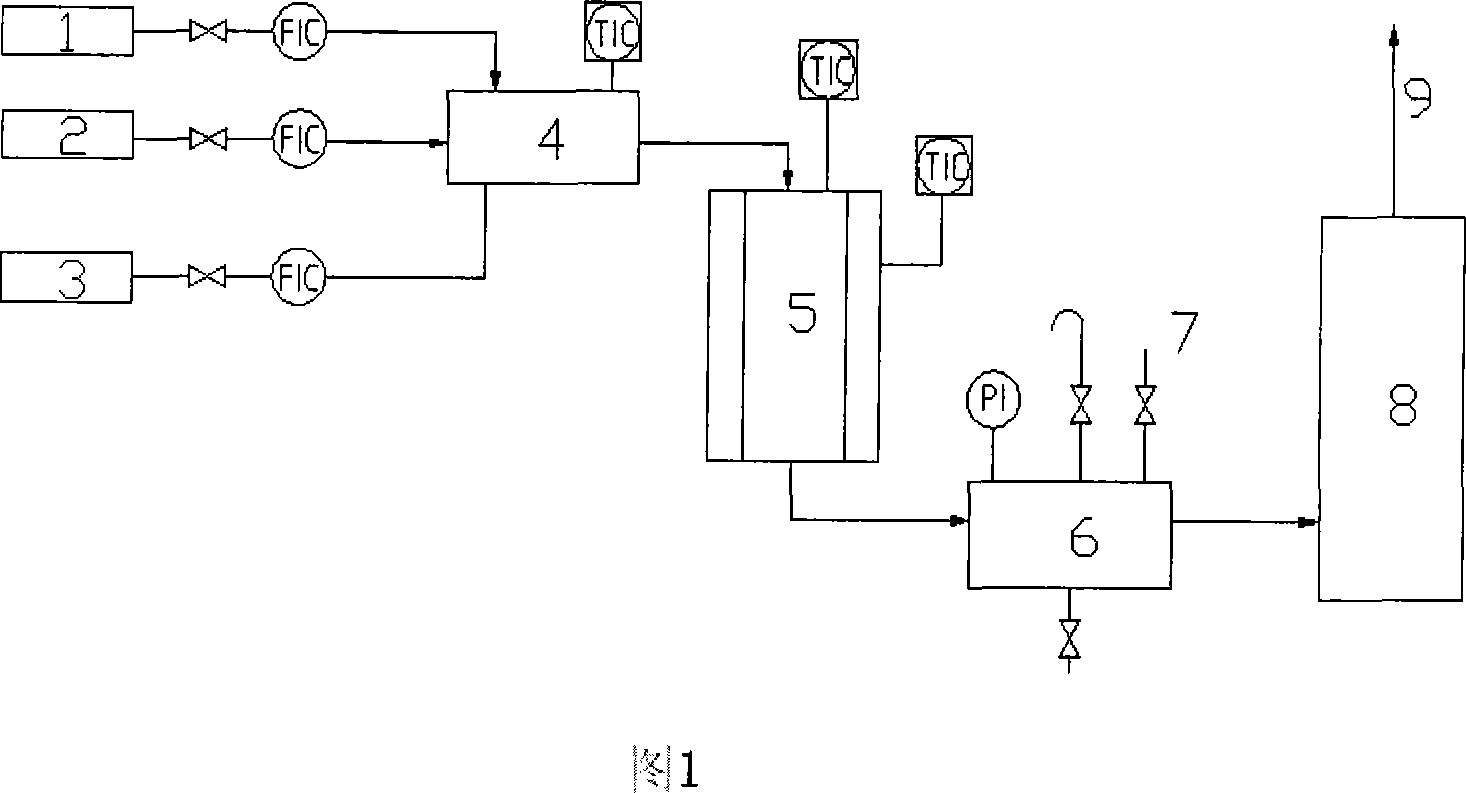
Catalyst for ammonia catalytic oxidation to nitrous oxide and applications thereof
A nitrous oxide catalyst and ammonium oxidation technology, which is applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, nitric oxide, etc., can solve the problem of high cost, explosion hazard, and high price of ammonium nitrate and other issues to achieve the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Composition is 8%MoO 3 / 2% Bi 2 o 3 / 10%C 0 O / 1%V 2 o 5 The rest is Al 2 o 3 catalyst.
[0029] Catalyst preparation process: NH 4 HVO 3 , ammonium tetramolybdate (ammonium metamolybdate), bismuth nitrate, and cobalt chloride are dissolved in oxalic acid solution, added with hydrated aluminum hydroxide, boiled into a slurry, and initially sintered in an electric furnace at a preliminary sintering temperature of 300 ° C, ball milled, and pure water added Kneading, extruding into Φ3mm-Φ5mm strips, and then sintering in an electric furnace at 550°C to obtain the catalyst. The specific surface area of the catalyst is about 10m 2 / g.
[0030] The catalyst is used for catalytic oxidation of ammonia to synthesize nitrous oxide. The reaction mixture is ammonia+air+nitrogen (volume ratio: 1 part of ammonia, 5 parts of air, 6 parts of nitrogen). The temperature is 340°C and the reaction time is 0.8 seconds. The conversion rate of ammonia was 90.1%, and the selectivi...
Embodiment 2
[0032] Composition is 15% MoO 3 / 5% Bi 2 o 3 / 8%C 0 O / 2%V 2 o 5 The rest is Al 2 o 3 catalyst. The catalyst preparation method is basically the same as in Example 1, except that the preliminary sintering temperature is 350°C. The specific surface area of the catalyst is about 58m 2 / g.
[0033] The catalyst is used for catalytic oxidation of ammonia to synthesize nitrous oxide. The reaction mixture is ammonia+air+nitrogen (1 part of ammonia, 5 parts of air, 6 parts of nitrogen, volume ratio), at a temperature of 340° C., and a reaction time of 1.5 seconds. The conversion rate of ammonia was 99.4%, and the selectivities of producing nitrous oxide and nitrogen oxide were 87% and 0.5%, respectively.
Embodiment 3
[0035] Composition is 20% MoO 3 / 5% Bi 2 o 3 / 5%C 0 O / 5%V 2 o 5 The rest is Al 2 o 3 catalyst. The catalyst preparation method is basically the same as in Example 1, except that the preliminary sintering temperature is 400°C. The specific surface area of the catalyst is about 40m 2 / g.
[0036] The catalyst is used for catalytic oxidation of ammonia to synthesize nitrous oxide. The reaction mixture is ammonia + air + nitrogen (1 part of ammonia, 5 parts of air, 6 parts of nitrogen, volume ratio), at a temperature of 340 ° C, and a reaction time of 2.3 seconds . The conversion rate of ammonia was 89.1%, and the selectivities of producing nitrous oxide and nitrogen oxide were 83% and 3.5%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com