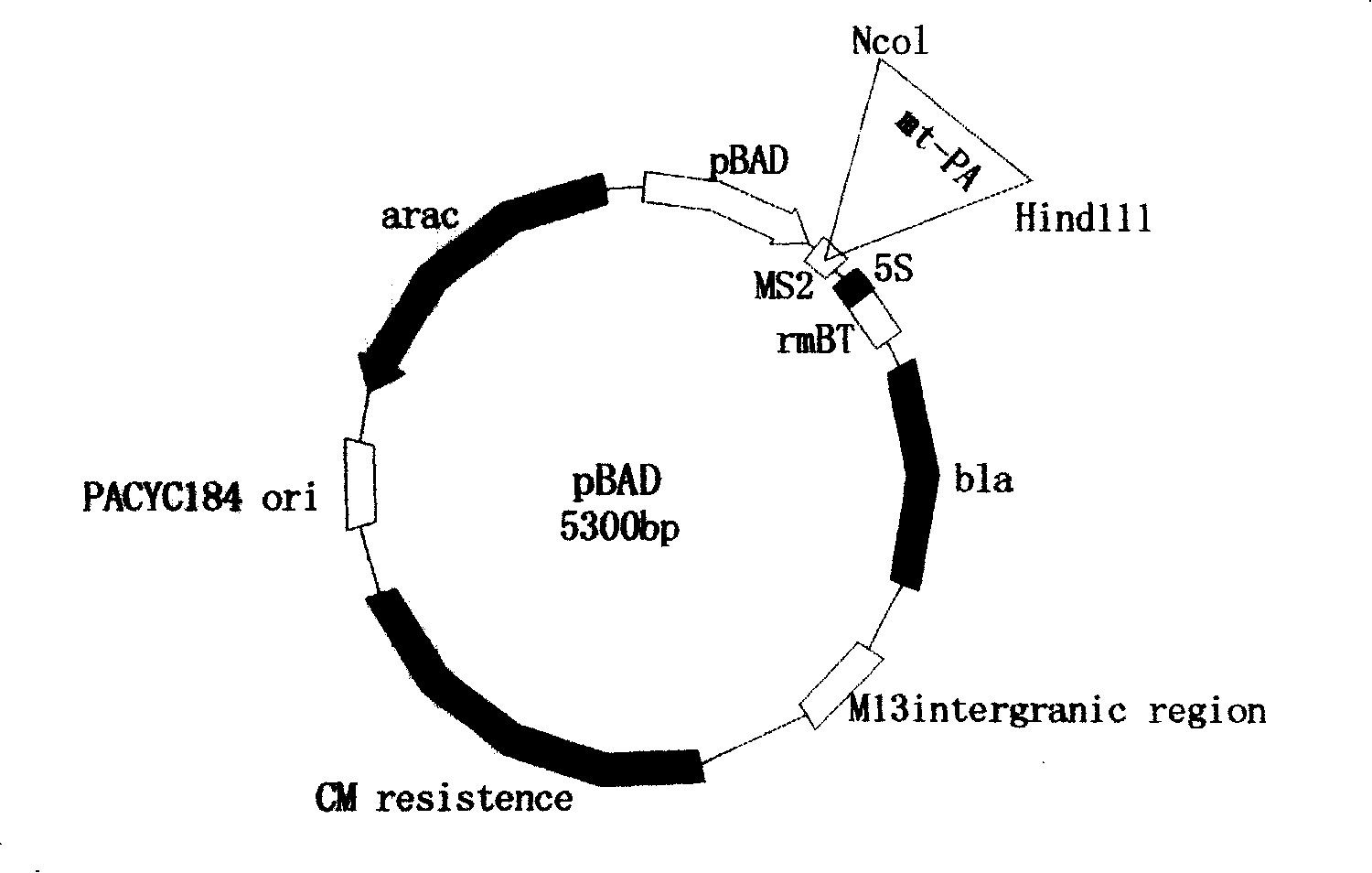
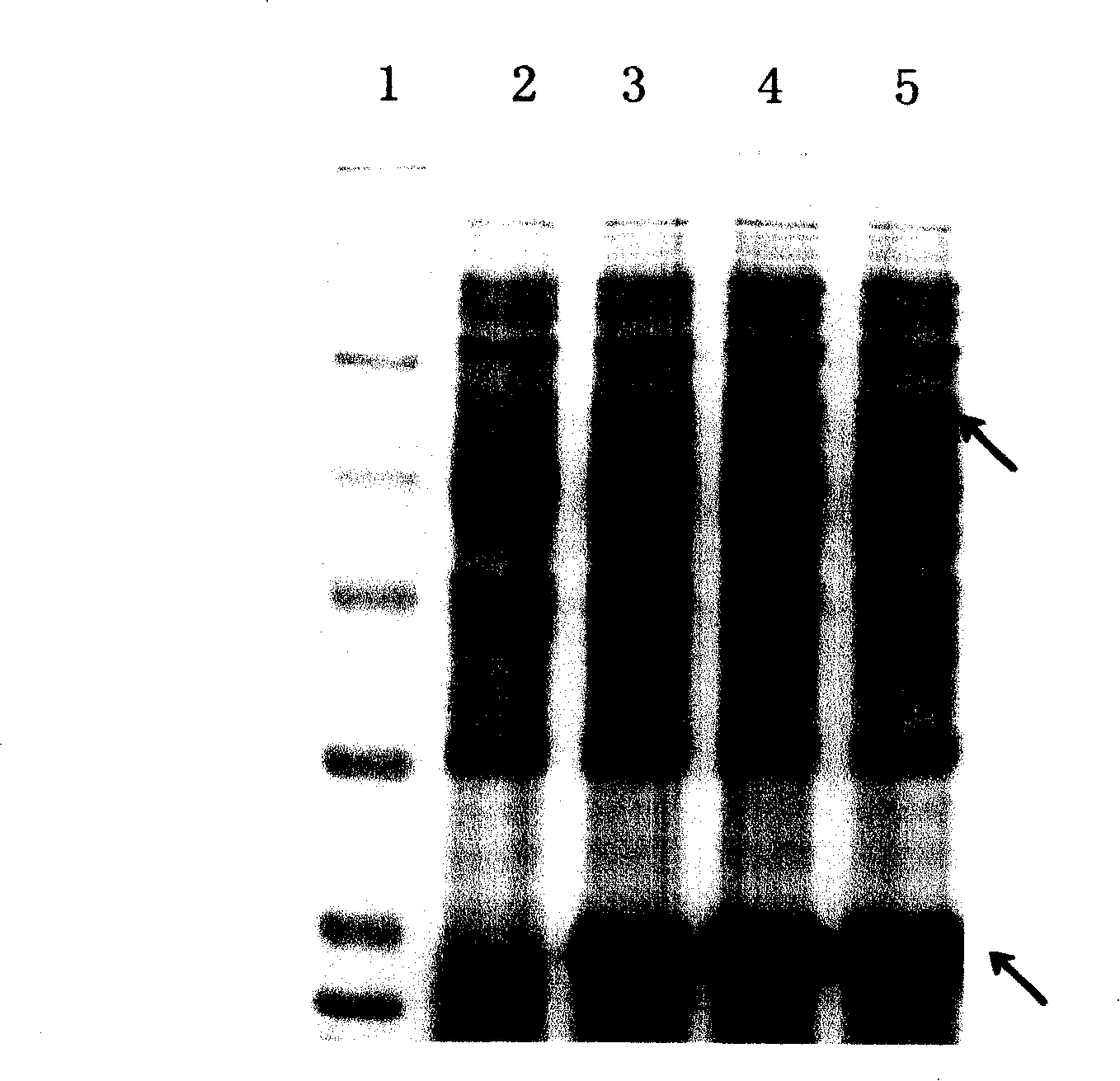
Technology for preparing active t-PA by procaryotic cells
A technology of prokaryotic cells and prokaryotic bacteria, applied in the field of high-efficiency expression, can solve the problems of high product cost, high price and low yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] 1. Mutation of wild-type human t-PA: the method is to use gene mutation technology to make the amino acid 296-299 in the wild-type protease function P region of wild-type human t-PA combined with plasmin activator inhibitor-1 (Lys-His-Arg-Arg) was mutated to (Ala-Ala-Ala-Ala). And a new endonuclease Not1 site was formed in the mutation region.
[0027] 1). Using wild-type human t-PA as a template, artificially synthesized primers 1, 2, 3 and 4 were used for PCR amplification, respectively.
[0028] Primers were designed as follows:
[0029] Primer 1: (27mer, and introduce Nco1 endonuclease site, beside the character box)
[0030] 5'-GCG gagc cagatcttac caag-3'
[0031] Primer 2: (34mer, and introduce Not1 endonuclease site, beside the character box)
[0032] 5'-ccgctccgggcgat ggcaaagat-3'
[0033] Primer 3: (34mer, and introduce Not1 endonuclease site, beside the character box)
[0034] 5'-ttgcc tcgcccggagagcggtt-3'
[0035] Primer 4: (33mer, and introduce ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com