Polythiourethane-based polymerizable composition and optical resin obtained from the same
A technology of polymeric composition and polythiol compound, which is applied in the field of resin and optical components, and polymeric composition, and can solve problems such as cracks in the coating film and poor dyeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0126] The preparation method of the resin of the present invention is explained below.
[0127] The resin of this invention is obtained by making said (A)-(C) component react. The mixing order of the ingredients is not particularly limited, for example, the following methods can be mentioned:
[0128] (i) A method in which (A) component and (C) component are reacted in advance, and (B) component is added and polymerized, and
[0129] (ii) A method of mixing (A), (B) and (C) components together and polymerizing them. In addition, as a polymerization method, for example, a heat curing method can be used.
[0130] When the component (C) is a compound that does not contain any ether bond or thioether bond in its molecular structure, the method (i) above is preferable. Thereby, it is possible to further suppress severe heat generation and rapid viscosity increase at the time of mixing the components.
[0131] The resin obtained by the method (i) or (ii) above has excellent imp...
Embodiment 1
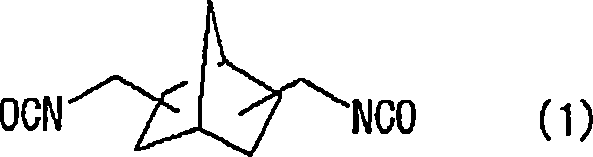
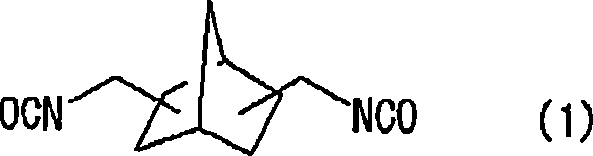
[0171] At 20°C, in 57.38g of 2,5(6)-bis(isocyanatomethyl)-bicyclo[2.2.1]heptane, 0.10g of dibutyltin chloride as a curing catalyst was mixed and dissolved, 0.25 g of an internal release agent (trade name: ZelecUN), and 0.05 g of an ultraviolet absorber (trade name: BioSorb583) were prepared into a homogeneous solution. After adding 7.52 g of 1,4-butanediol to this homogeneous solution and stirring at 30° C. for 20 minutes, it was cooled to 20° C. and stirred for an additional 30 minutes. Then, 3 to 5.10 g of 4-mercaptomethyl-1,8-dimercapto-3,6-dithiaoctane were added, mixed and dissolved.
[0172] After defoaming the homogeneous solution at 600 Pa for 1 hour, it was filtered through a filter made of 1 μm PTFE, and injected into an injection mold composed of a glass mold and a tape. The injection mold was put into an oven, and the temperature was gradually raised from 25°C to 120°C over 20 hours to carry out polymerization. After the polymerization is completed, the injection...
Embodiment 2
[0175] At 20°C, 0.10 g of dibutyltin chloride, 0.25 g of an internal release agent (trade name: ZelecUN), and 0.05 g of an ultraviolet absorber (trade name: BioSorb583) were prepared into a homogeneous solution. After adding 7.89 g of triethylene glycol to this homogeneous solution and stirring at 30° C. for 20 minutes, it was cooled to 20° C. and then stirred for 30 minutes. Then, 37.89 g of 4-mercaptomethyl-1,8-dimercapto-3,6-dithiaoctane was added, mixed and dissolved.
[0176] After defoaming the homogeneous solution at 600 Pa for 1 hour, it was filtered through a filter made of 1 μm PTFE, and injected into an injection mold composed of a glass mold and a tape. The injection mold was put into an oven, and the temperature was gradually raised from 25°C to 120°C over 20 hours to carry out polymerization. After the polymerization is completed, the injection mold is taken out from the oven, and the resin is obtained by demoulding. The resulting resin was further annealed at...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal resistance | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thermal resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com