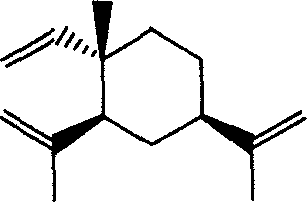
Beta-elemene diamine derivatives, synthesis method and use thereof
A technology of elemene diamine and synthesis method, which is applied in the direction of amine active ingredients, drug combination, chemical instrument and method, etc., can solve the problems of poor water solubility and limited clinical application, and achieve good water solubility and good antitumor drug high efficacy and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~9
[0038] Embodiment 1~9β-elemene diamine derivative
[0039] Table 1 shows Examples 1-9 of β-elemene diamine derivatives with the structural formula shown in Formula I.
[0040] Formula I
[0041] Table 1 Examples 1-9 of β-elemene diamine derivatives
[0042]
[0043] The synthesis of reference example β-elemene chloride
[0044] According to the method of literature (Jia Weimin, synthesis, structure and structure-activity research of new anticancer drug β-elemene and its derivatives, doctoral thesis of Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 1991), the method for synthesizing β-elemene chloride, specifically Proceed as follows:
[0045]Dissolve 0.01mol β-elemene in 10mL of dichloromethane, add 2mL of formic acid, control the temperature at 0-5°C, slowly add 15mL of sodium hypochlorite solution dropwise within 2 hours, and continue the reaction for 3-5 hours. After the reaction is complete, add saturated aqueous sodium bicarbonate solution to ...
Embodiment 2
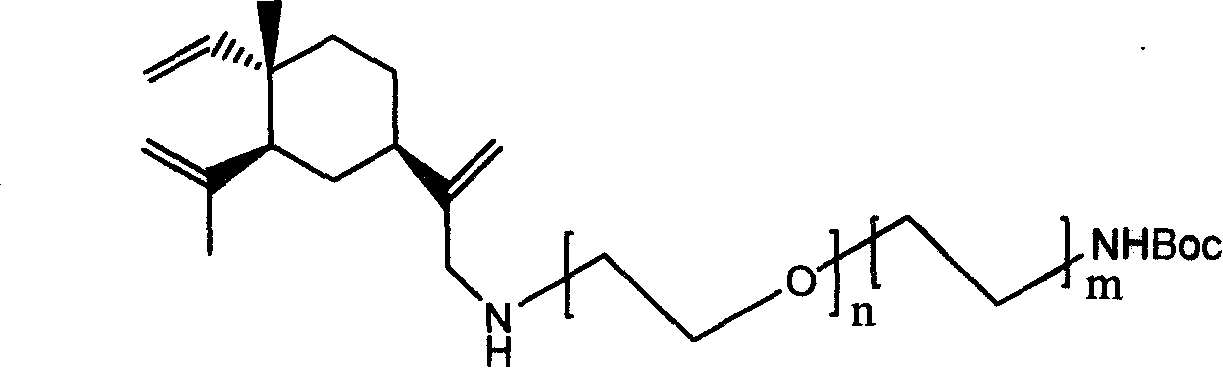
[0052] Method Example 2 β-elemene monosubstituted hexamethylenediamine derivative (compound of formula I, wherein n=0, m=3)
[0053]
[0054] The β-elemene chlorinated mixture was mixed with NH 2 (CH 2 CH 2 ) 3 NHBoc was dissolved in acetonitrile and Na 2 CO 3 . β-elemene chloride and NH 2 (CH 2 CH 2 ) 3 The molar ratio of NHBoc is 1:2; 2 CO 3 The molar ratio is 1:10. It can be reacted for 3 hours at 120°C. The identification results are as follows:
[0055] 1 H-NMR (CDCl 3 , TMS, 400MHz), δ: 0.99(s, 3H), 1.25-1.80(m, 15H), 1.44(s, 9H), 1.71(s, 3H), 2.01-2.11(m, 1H), 2.97(t , J=7.65, 2H), 3.08(q, J=6.34, 2H), 3.64(s, 2H), 4.58(s, 1H), 4.82(s, 1H), 4.88(s, 1H), 4.92(d , J=3.74, 1H), 5.18(s, 1H), 5.23(s, 1H), 5.80(dd, J=17.76, 10.54, 1H).
Embodiment 3
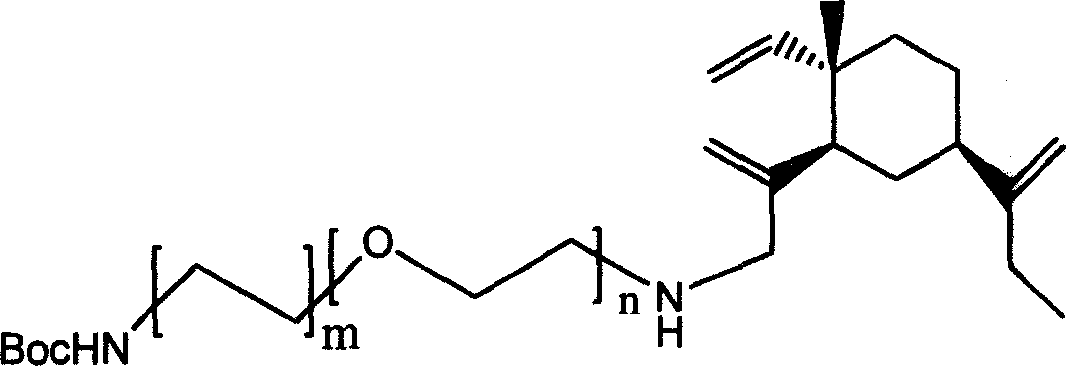
[0056] Method Example 3 Compound of Formula I (n=2, m=1)
[0057]
[0058] The β-elemene chlorinated mixture was mixed with NH 2 (CH 2 CH 2 O) 2 CH 2 CH 2 NHBoc was dissolved in acetonitrile, KOH and XI were added. β-elemene chloride and NH 2 (CH 2 CH 2 O) 2 (CH 2 CH 2 ) 1 The molar ratio of NHBoc is 1:3; the molar ratio to KOH is 1:5; the molar amount of KI is 5% of the molar amount of β-elemene chloride. React at 70°C for 15 hours, and the identification results are as follows:
[0059] 1 H-NMR (CDCl 3 , TMS, 500MHz), δ: 1.00(s, 3H), 1.30-1.53(m, 10H), 1.44(s, 9H), 1.53-1.65(m, 2H), 1.71(s, 3H), 1.85-1.92 (m, 2H), 2.08-2.23(m, 2H), 2.88(t, J=7.5, 2H), 3.08(d, J=4.61, 2H), 3.54-3.63(m, 2H), 4.58(s, 1H), 4.83(s, 1H), 4.89(s, 1H), 4.91(d, J=6.66, 1H), 5.26(s, 1H), 5.35(s, 1H), 5.82(dd, J=17.29, 11.11, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com