Cyclosporins A emulsion preparations for eyes and preparation thereof
A technology of cyclosporine and cyclosporine, which is applied in the direction of medical preparations containing active ingredients, cyclic peptide components, and pharmaceutical formulas, and can solve problems such as high process requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
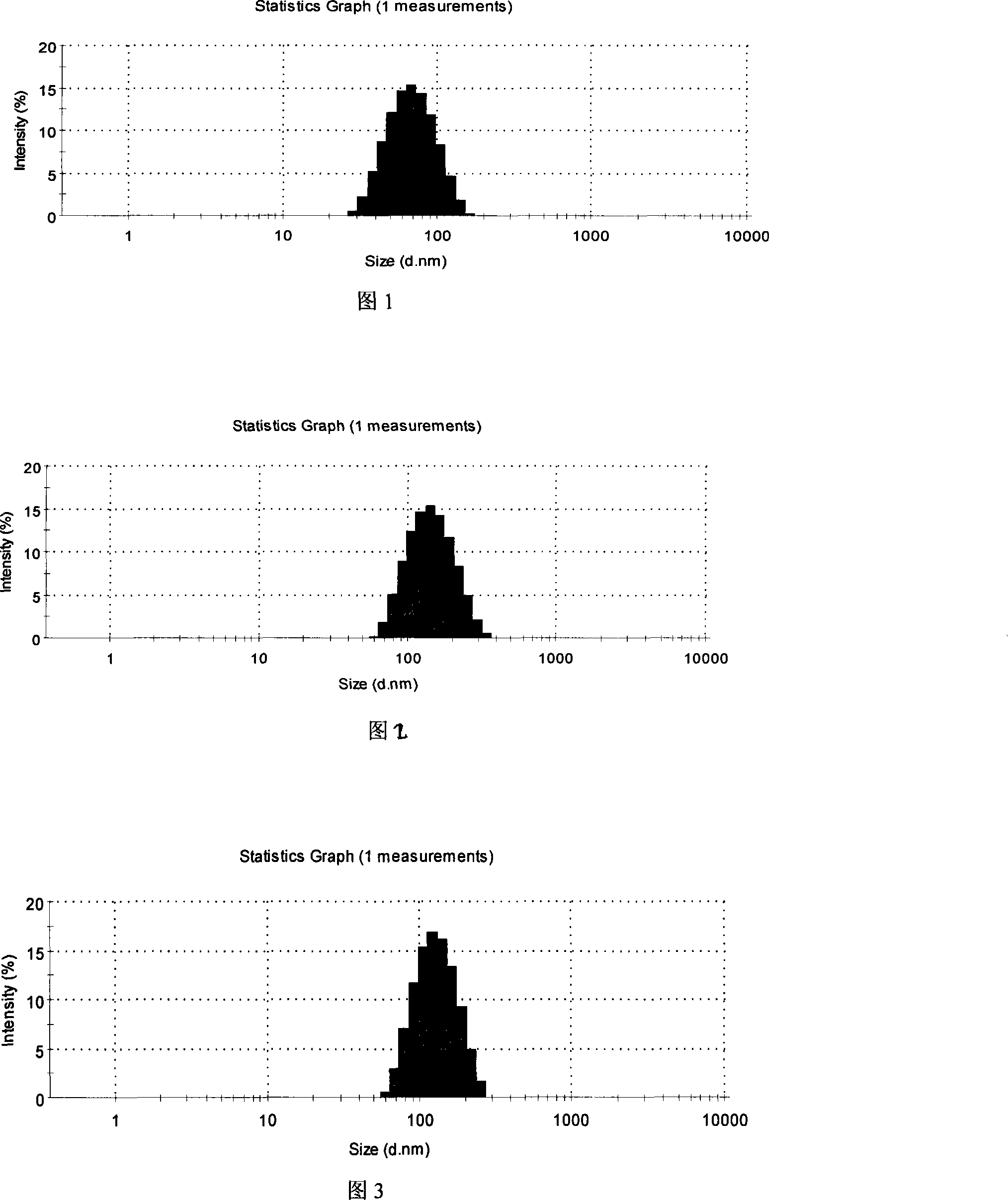
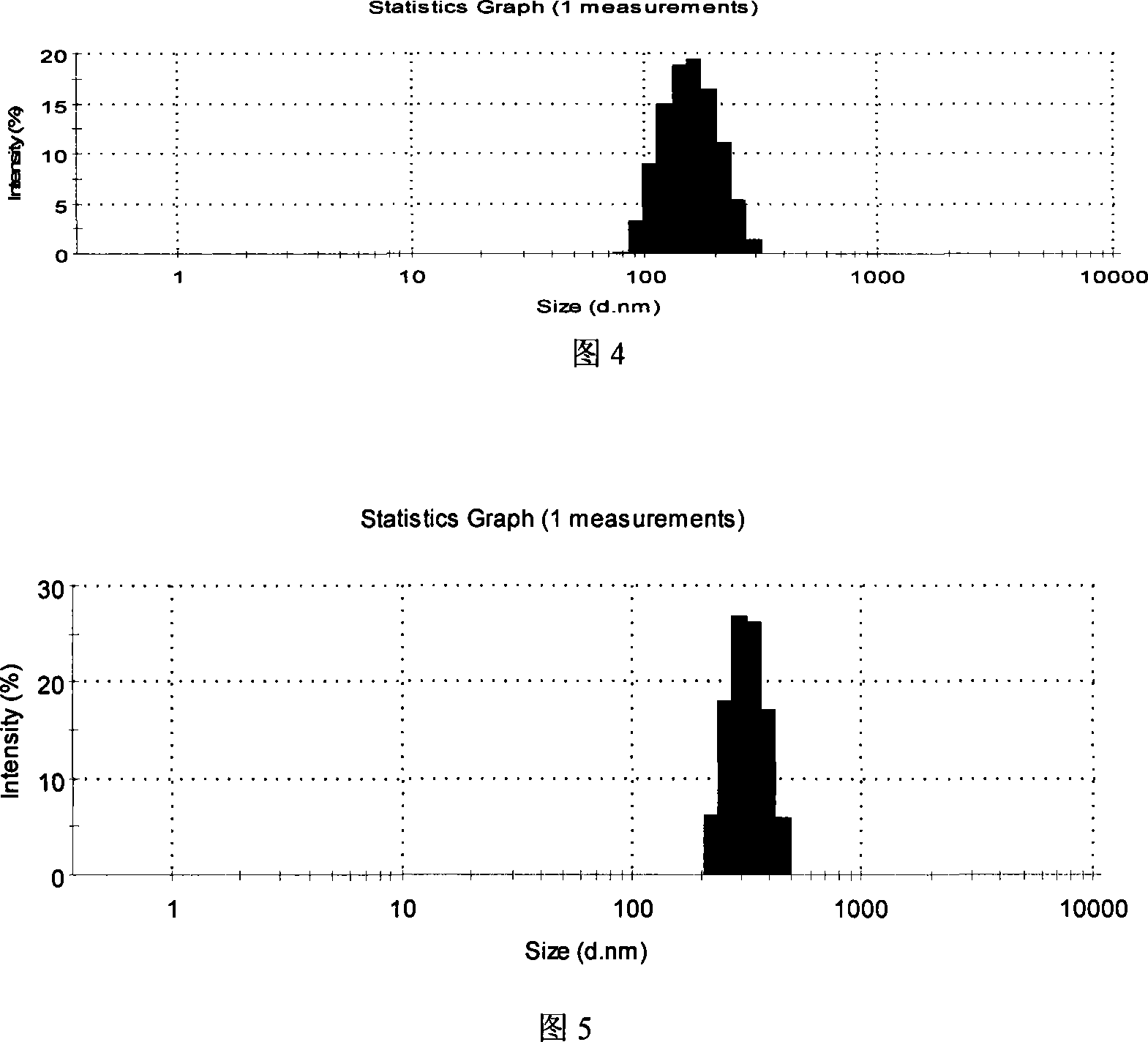
Image
Examples
Embodiment 1
[0047] A
[0048] Preparation method: Weigh each component according to the composition of the prescription, and follow the operation steps as 1) steam distill 100g of chrysanthemum or 60g of honeysuckle, collect 1000ml of distillate, separate the upper oily liquid, concentrate the remaining liquid to 400ml, and mix with the oily liquid Mix well; 2) Put hydroxypropyl methylcellulose (HPMC) into appropriate amount of water, heat to 80-90°C, stir, disperse and cool to room temperature; 3) Add appropriate amount of chrysanthemum and honeysuckle concentrate to HPMC solution 4) Dissolve cyclosporine A in the oil phase; 5) Mix evenly with emulsifier and glycerin; 6) Slowly add the mixture obtained in 3) to the obtained cyclosporin A mixture , ultrasonic shear emulsification, 1 to 5 minutes; 7) Use a pH regulator to adjust the pH value to 7.2 to 7.8; 8) Add the prescribed amount of purified water to make up to a sufficient amount, and then filter it with a 0.45um sterile...
Embodiment 2
[0050] A
[0051] Preparation method: weigh each component according to the composition of the prescription, steam distill 50g of chrysanthemum and 20g of honeysuckle according to step 1), collect 1000ml of distillate, separate the upper oily liquid, concentrate the remaining liquid to 400ml, and mix well with the oily liquid ,; 2) Put hydroxypropyl methylcellulose (HPMC) into an appropriate amount of water, heat to 80-90°C, stir, disperse and cool to room temperature; 3) Add appropriate amount of chrysanthemum and honeysuckle concentrate to the HPMC solution, Mix well and set aside; 4) Dissolve cyclosporin A in the oil phase; 5) Mix evenly with emulsifier and glycerin; 6) Slowly add the mixture obtained in 3) to the obtained cyclosporine A mixture, and ultrasonically Shear emulsification, 1-5 minutes; 7) Use a pH regulator to adjust the pH value to 7.2-7.8; 8) Add the prescribed amount of purified water to make up to a sufficient amount, and then filter through a...
Embodiment 3
[0053] A
[0054] Preparation method: weigh each component according to the composition of the prescription, steam distill 50g of chrysanthemum and 20g of honeysuckle according to step 1), collect 1000ml of distillate, separate the upper oily liquid, concentrate the remaining liquid to 400ml, and mix well with the oily liquid ,; 2) Put hydroxypropyl methylcellulose (HPMC) into an appropriate amount of water, heat to 80-90°C, stir, disperse and cool to room temperature; 3) Add appropriate amount of chrysanthemum and honeysuckle concentrate to the HPMC solution, Mix well and set aside; 4) Dissolve cyclosporin A in the oil phase; 5) Mix evenly with emulsifier and glycerin; 6) Slowly add the mixture obtained in 3) to the obtained cyclosporine A mixture, and ultrasonically Shear emulsification, 1-5 minutes; 7) Use a pH regulator to adjust the pH value to 7.2-7.8; 8) Add the prescribed amount of purified water to make up to a sufficient amount, and then filter through a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com