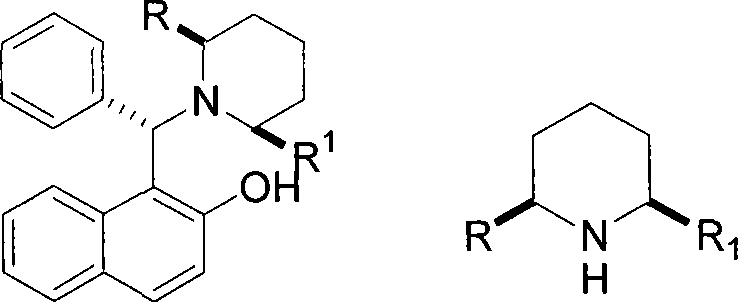
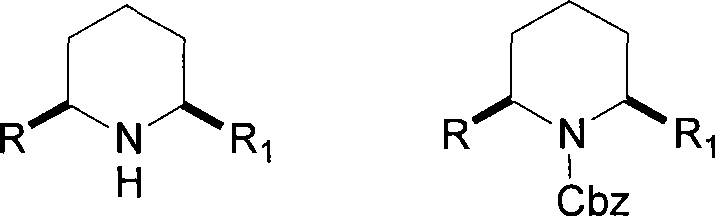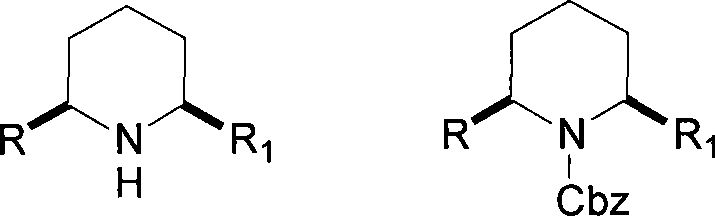Method for preparing chiral alpha-alkenyl(or alkynyl group)-alpha'-alkyl piperidine as well as N-Cbz derivatives and use thereof
A technology based on alkyl and alkenyl, applied in the field of preparation and application of chiral α-alkenyl (or alkynyl)-α'-alkylpiperidine and its N-Cbz derivatives, achieving high yield and easy operation easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Embodiment 1, the preparation of (2R, 6R)-2-n-propyl-6-vinylpiperidine-1-benzyl carbonate (formula VI-a)
[0085]
[0086] A solution of compound formula IV-a (1.0 g, 2.59 mmol) with aqueous sodium hydroxide solution (6M, 1 mL), methanol (2 mL) and tetrahydrofuran (2 mL) was stirred and reacted at 60° C. for 2.0 hours. Then the system was cooled to room temperature and washed with CH 2 Cl 2 Moderately extract three times. The organic phases were combined and extracted with aqueous HCl (6.0 M, 5 x 5 mL) until compound V-a was completely salted and dissolved in the aqueous phase. Combine the aqueous phases with CH 2 Cl 2 Extract (3 x 10 mL) and discard the organic phase. Add NaOH aqueous solution (6.0M) dropwise to the remaining aqueous phase until the solution is alkaline (pH=10-12), and then use CH 2 Cl 2 Moderately extract three times. The combined organic phase containing compound formula V-a was washed with saturated brine, anhydrous NaSO 4 dry.
[0087]...
Embodiment 2
[0092] Embodiment 2, the preparation of (2R, 6R)-2-n-pentyl-6-vinylpiperidine-1-benzyl carbonate (formula VI-b)
[0093]
[0094] A solution of compound formula IV-b (1.0 g, 2.42 mmol), sodium hydroxide aqueous solution (6M, 1 mL), methanol (2 mL) and tetrahydrofuran (2 mL) was stirred and reacted at 60° C. for 2.0 hours. Then the system was cooled to room temperature and separated by column chromatography (silica gel, PE / EtOAc).
[0095] After evaporating most of the solvent from the resulting eluent containing compound formula V-b, add CH 2 Cl 2(20mL), Na 2 CO 3 (1.28g, 12.10mmol) and CbzCl (0.83g, 4.84mmol), mixed and stirred at room temperature for 5h. The system was cooled to room temperature, filtered to remove Na 2 CO 3 , the crude product obtained after evaporating the solvent was subjected to column chromatography (silica gel, PE / EtOAc) to obtain oily liquid formula VI-b (0.63 g, 82%).
[0096] [α] D 25 =+25.8° (c 1.5, CHCl 3 ).
[0097] 13 C NMR: δ 156...
Embodiment 3
[0100] Embodiment 3, the preparation of (2R, 6R)-2-n-hexyl-6-vinylpiperidine-1-benzyl carbonate (formula VI-c)
[0101]
[0102] Under the same conditions as in Example 2, the product formula VI-c was obtained from the compound formula IV-c with a yield of 80%.
[0103] [α] D 25 =+28.3° (c 1.4, CHCl 3 ).
[0104] 13 C NMR: δ 156.0, 139.7, 137.0, 128.4, 127.8, 114.8, 66.9, 51.7, 51.1, 34.1, 31.8, 29.2, 27.8, 27.7, 27.1, 22.6, 14.5, 14.1.
[0105] Elemental Analysis: Calcd.for C 21 h 31 NO 2 : C, 76.55; H, 9.48; N, 4.25. Found: C, 77.02; H, 9.41; N, 4.20.
[0106] Indicates that the obtained compound is correct.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com