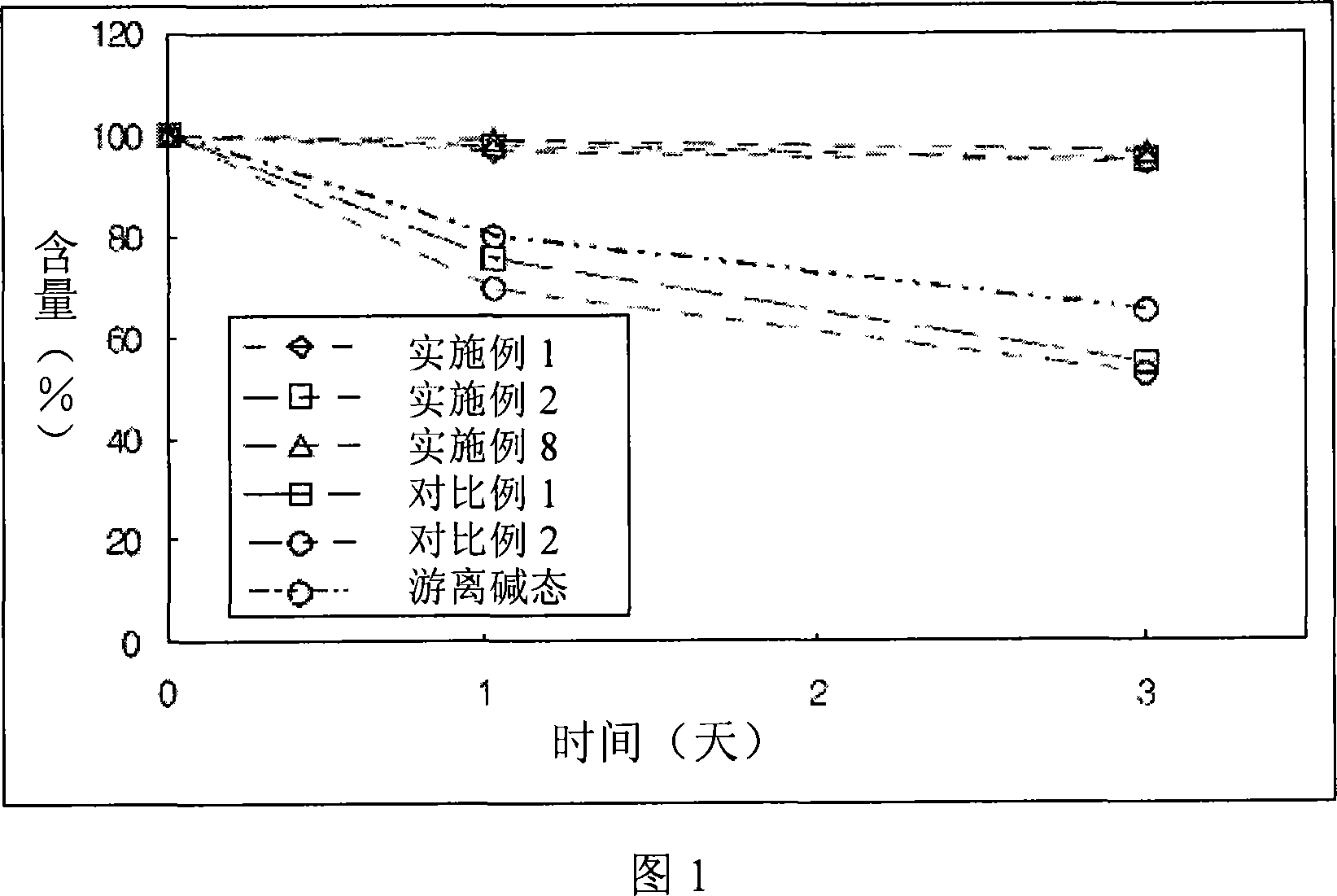
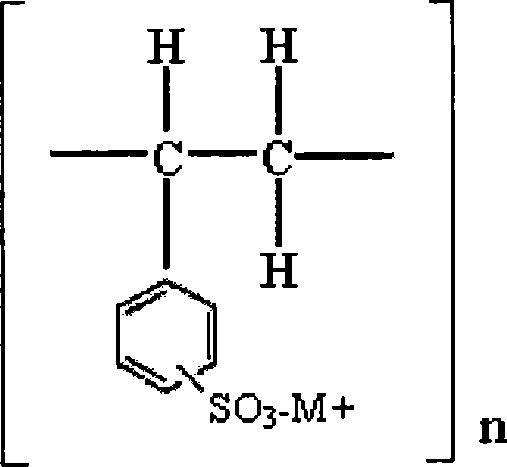
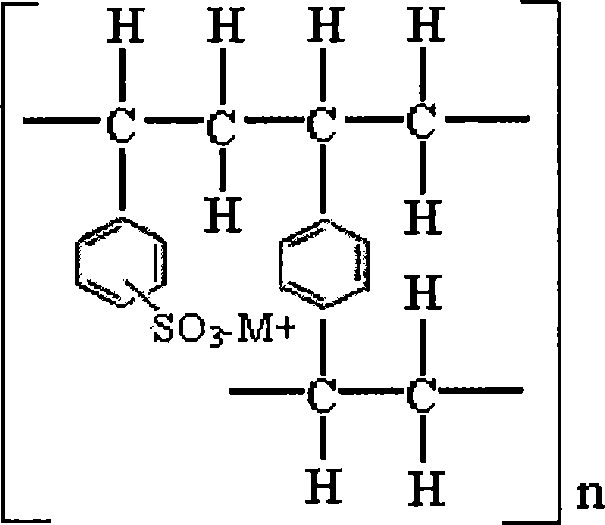
Novel resinate complex of S-clopidogrel and production method thereof
A technology of clopidogrel and resinate, applied in the directions of non-active ingredient medical preparations, active ingredients-containing medical preparations, drug combinations, etc., can solve problems such as affecting the stability of esters, achieve good safety, excellent The effect of liquidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1: Methyl (+)-(S)-(2-chlorophenyl)(6,7-dihydro-4H-thieno[3,2-c]pyridin-5-yl)acetate- Styrene Sulfonate Polymer Compound
[0083] 2.40 g (7.45 mmole) of (+)-clopidogrel isomer (free base) was dissolved in 50 ml of acetone, cooled and stirred. 1.25 mL of 18% styrene sulfonate polymer solution was slowly added dropwise to the cooled solution. The resulting solution was stirred for a period of time during which time a precipitate formed. After decanting the supernatant of the solution, 20 mL of cooled acetone was added and stirred. The solid precipitate was then collected by filtration and dried in a vacuum oven. In the collected resinate complexes, approximately 54.2% of the total dry solids were (+)-clopidogrel isomers as determined by high performance liquid chromatography (HPLC) analysis.
Embodiment 2
[0084] Example 2: Methyl (+)-(S)-(2-chlorophenyl)(6,7-dihydro-4H-thieno[3,2-c]pyridin-5-yl)acetate- Styrene Sulfonate Polymer Compound
[0085] 0.44 g (1.35 mmole) of (+)-clopidogrel isomer (free base) was dissolved in 50 ml of acetone, cooled and stirred. 1.2 mL of 18% styrene sulfonate polymer solution was slowly added dropwise to the cooled solution. The resulting solution was stirred for a period of time during which time a precipitate formed. After decanting the supernatant of the solution, 20 mL of cooled acetone was added and stirred. The solid precipitate was then collected by filtration and dried in a vacuum oven. As determined by HPLC analysis, about 52.1% of the total dry solids in the collected resinate complexes were (+)-clopidogrel isomers.
Embodiment 3
[0086] Example 3: Methyl (+)-(S)-(2-chlorophenyl)(6,7-dihydro-4H-thieno[3,2-c]pyridin-5-yl)acetate- Styrene Sulfonate Polymer Compound
[0087] 2.40 g (7.45 mmole) of (+)-clopidogrel isomer (free base) was dissolved in 100 ml acetone, cooled and stirred. 1.2 mL of 18% styrene sulfonate polymer solution was slowly added dropwise to the cooled solution. The resulting solution was stirred for a period of time during which time a precipitate formed. After decanting the supernatant of the solution, 50 mL of cooled acetone was added and stirred. The solid precipitate was then collected by filtration and dried in a vacuum oven. As determined by HPLC analysis, about 53.6% of the total dry solids in the collected resinate complexes were (+)-clopidogrel isomers.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com