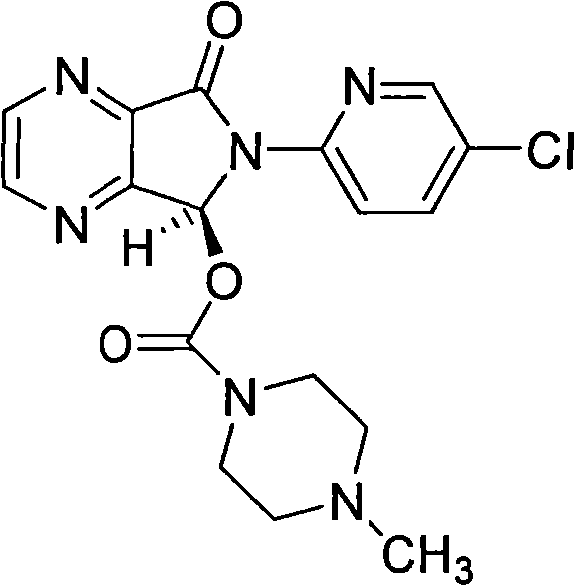
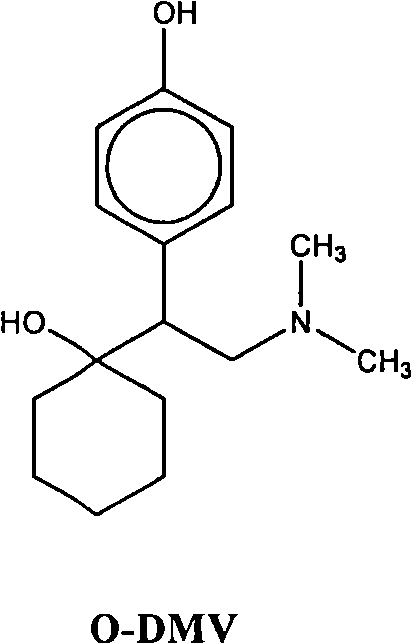
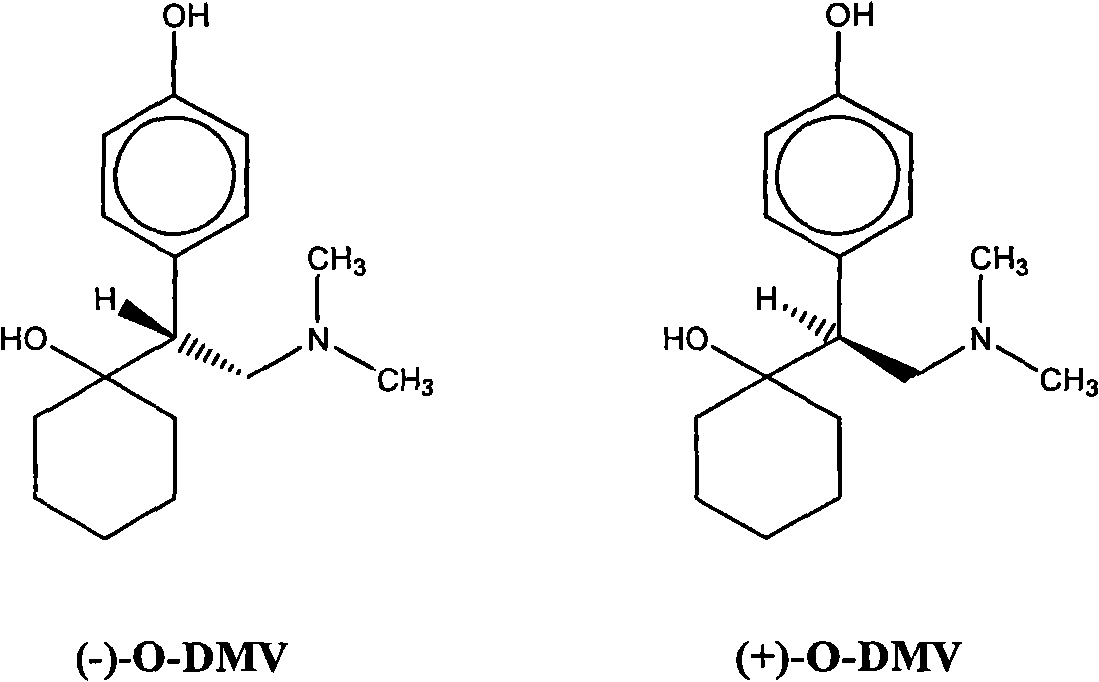
Combinations of eszopiclone and O-desmethylvenlafaxine, and methods of treatment of menopause and mood, anxiety, and cognitive disorders
A technology for eszopiclone and mood disorders, applied in the composition of anxiety disorders and cognitive disorders, in the field of treatment of menopause and mood disorders, and can solve problems such as inability to improve cognitive function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Example 1. Dosage Form
[0115] The following dosage form is an example of a combination tablet or capsule of eszopiclone and O-desmethylvenlafaxine:
[0116] Table 1. Combination dosage forms of eszopiclone and (-)-O-DMV
[0117]
[0118] Table 2. Combination Dosage Forms of Eszopiclone and Racemic O-DMV
[0119]
[0120]
[0121] * 1.0 mg O-DMV free base is equivalent to 1.517 mg succinate monohydrate salt.
[0122] Above-mentioned dosage form can carry out following steps to prepare:
[0123] 1. Screening eszopiclone by 80 mesh.
[0124] 2. Screen O-DMV through 40 mesh.
[0125] 3. Sift remaining ingredients through #20 or #30 mesh sieve.
[0126] 4. Mix eszopiclone and part of MCC (microcrystalline cellulose).
[0127] 5. Mix O-DMV with the mixture from step 4.
[0128] 6. Mix the mixture from step 5 with the remaining MCC from step 3.
[0129] 7. Mix the mixture from step 6 with the dicalcium phosphate.
[0130] 8. Mix the croscarmellose with the s...
Embodiment 2
[0135] Example 2. Clinical research on the treatment of menopause or perimenopause with eszopiclone
[0136] The aim of the study was to observe the efficacy of eszopiclone 3 mg compared with placebo in the treatment of insomnia secondary to perimenopause or menopause.
[0137] The study was a multicenter, randomized, double-blind, placebo-controlled, parallel-group study. The study had a one-week single-blind placebo enrollment period, followed by four weeks of double-blind treatment and a one-week single-blind placebo wash-out. The preliminary method of analysis compared post-randomization results between the two treatment groups.
[0138] The subjects were women with insomnia secondary to perimenopause or menopause. Subjects are pre-menopausal or menopausal and suffer from insomnia symptoms, including sleep latency (SL) ≥ 45 minutes and total sleep time (TST) ≤ 6 hours. Perimenopausal / menopausal symptoms precede sleep disturbance symptoms. The patient population was pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com