Method for preparing trihydrated magnesium carbonate from magnesium chloride-containing bittern by using ammonium carbonate
A technology of magnesium carbonate trihydrate and magnesium chloride, which is applied in the field of salt chemical industry, can solve the problems of difficult control of the reaction process and unstable product quality, and achieve the effect of optimizing the precipitation and crystallization process, good crystal form, and reducing the adsorption of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
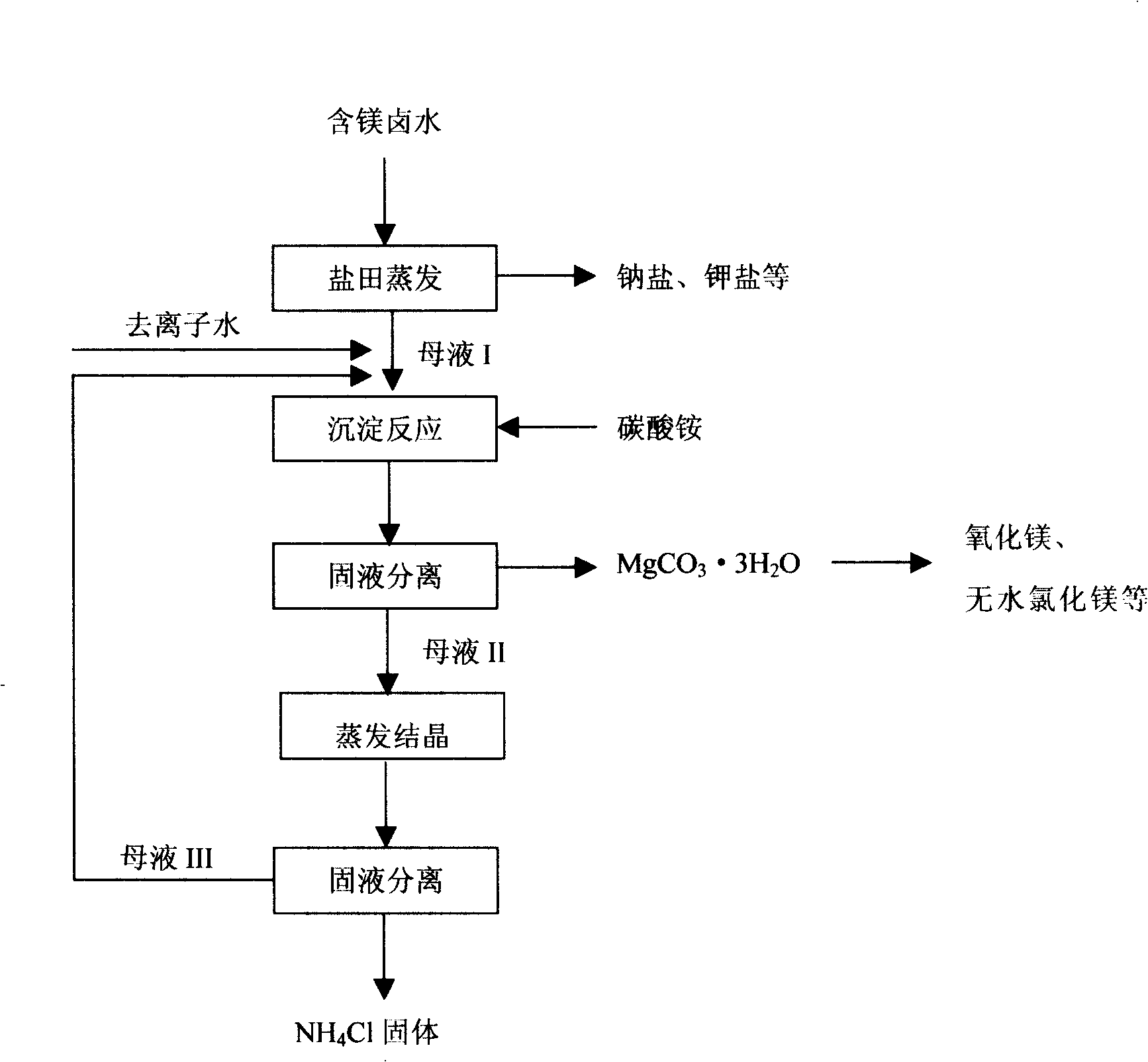
[0045] The raw brine containing magnesium chloride is naturally evaporated in the salt pan to obtain crystallization mother liquor containing magnesium chloride, and the refined saturated magnesium chloride brine with high magnesium content is obtained through precipitation and filtering impurities.
[0046] Add saturated magnesium chloride brine to the magnesium carbonate crystallizer, add deionized water to the brine containing magnesium chloride, and prepare 1000ml of magnesium chloride reaction bottom solution with a concentration of 1mol / L, heat to keep the solution at 30°C, and mix 1000ml with a concentration of 1.1 The mol / L ammonium carbonate solution was added slowly, and the adding speed was controlled so that the pH value of the reaction solution was between 8.3 and 8.5. After the reaction, continue to stir for 2 hours, and age to generate needle-shaped magnesium carbonate trihydrate precipitates. Wash the above precipitate with deionized water at 30°C, filter and d...
Embodiment 2
[0048] The raw brine containing magnesium chloride is naturally evaporated in the salt pan to obtain crystallization mother liquor containing magnesium chloride, and the refined saturated magnesium chloride brine with high magnesium content is obtained through precipitation and filtering impurities.
[0049] Add saturated magnesium chloride brine to the magnesium carbonate crystallizer, add deionized water to the brine containing magnesium chloride, and prepare 1000ml of magnesium chloride reaction bottom liquid with a concentration of 0.5mol / L, heat to keep the solution at 30°C, and dissolve 53g of ammonium carbonate under stirring The solid powder is slowly added therein, and the adding speed is controlled so that the pH value of the reaction solution is between 7.5 and 9.0. After the reaction was completed, the stirring was continued for 6 hours, and the needle-shaped magnesium carbonate trihydrate precipitate was formed by aging. Wash the above precipitate with deionized w...
Embodiment 3
[0051] The raw brine containing magnesium chloride is naturally evaporated in the salt pan to obtain crystallization mother liquor containing magnesium chloride, and the refined saturated magnesium chloride brine with high magnesium content is obtained through precipitation and filtering impurities.
[0052] Add saturated magnesium chloride brine to the magnesium carbonate crystallizer, add deionized water to the brine containing magnesium chloride, and prepare 1000ml of magnesium chloride reaction bottom solution with a concentration of 2mol / L, heat to keep the solution at 25°C, and mix 2100ml with a concentration of 1mol under stirring. / L of ammonium carbonate solution made of ammonium bicarbonate and ammonia water is slowly added to the crystallizer, and the addition speed is controlled so that the pH value of the reaction solution is between 8.3 and 8.5. After the reaction was completed, the stirring was continued for 6 hours, and the needle-shaped magnesium carbonate trih...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com