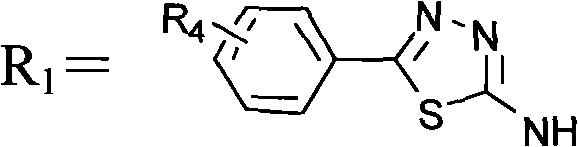
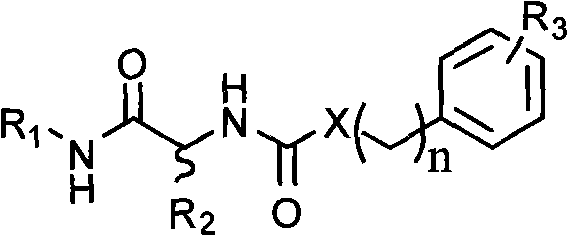
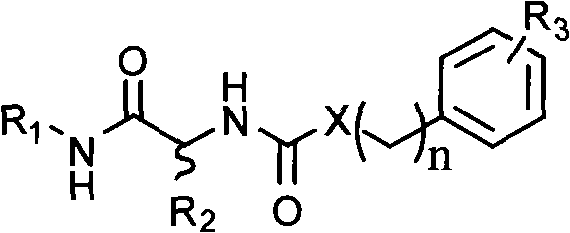Matrix metalloprotease inhibitors and synthetic method thereof
A technology of protease inhibitors and matrix metals, applied in the field of matrix metalloproteinase inhibitors and synthesis, to achieve the effects of short synthesis process routes, easy availability of raw materials, and low production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0025] Synthesis of benzenesulfonyl-D-alanine:
[0026]
[0027] Add 2.56ml (20mmol) of benzenesulfonyl chloride to 1.96g (22mmol) of D-alanine in Na 2 CO 3 solution (4.45gNa 2 CO 3 Dissolve in 30ml of water), under vigorous stirring, react at 70°C for 0.5hr. After the reaction was completed, cool in an ice bath, adjust the pH value to 2.5 with concentrated hydrochloric acid, put it in the refrigerator overnight, and precipitate a white precipitate, filter, wash the filter cake with a small amount of ice water, recrystallize with distilled water, and dry to obtain 1.58 g of the target product. : 34.5%, mp: 126-128°C, [α]=+32.2° (0.025g / ml methanol solution, room temperature) (R)-5-phenyl-N-((2-benzenesulfonamide)-1- Synthesis of oxy-propyl)-1,3,4-thiadiazol-2-amine
[0028]
[0029] Dissolve 1.37g (6mmol) benzenesulfonyl-D-alanine and 0.69g (6mmol) N-hydroxysuccinimide in dioxane, add DCC dioxane solution at room temperature, and react at room temperature for 4hr ,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com