Preparation of quaternaries hyper branched polycation electrolyte
A technology of cationic electrolytes and quaternary ammonium salts, applied in the field of hyperbranched polycationic electrolytes, can solve problems such as unrealizable, and achieve the effect of environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: Utilize SCATRP of MEBDAB to prepare hyperbranched polycation electrolyte PMEBDAB
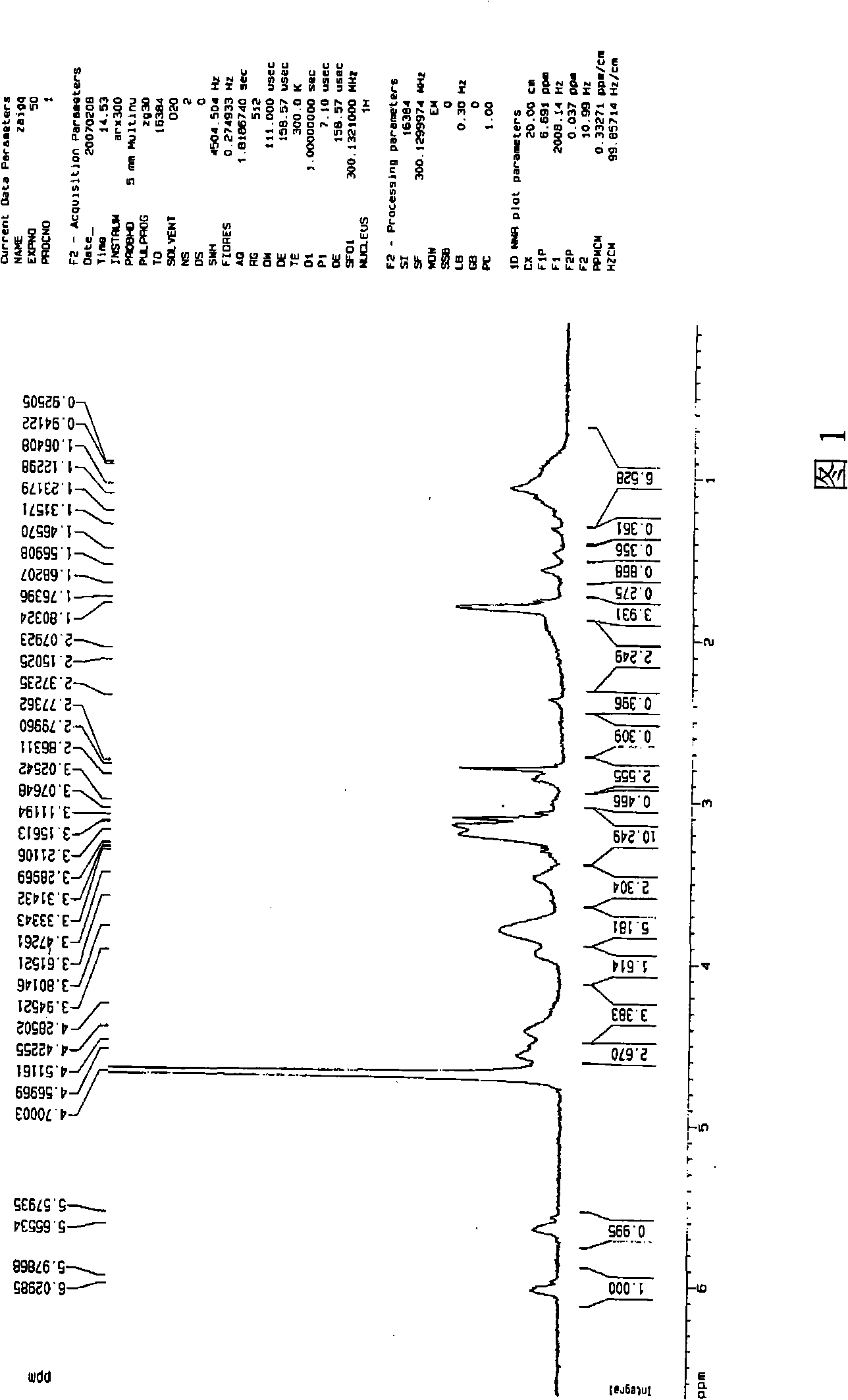
[0044] Add MEBDAB (4.31g, 10mmol), CuBr (14.4mg, 0.1mmol), PMDETA (34.6mg, 0.2mmol) and 8ml of water / ethanol (volume ratio 1 / 1) into a round bottom flask, repeatedly evacuate and fill with argon , and then sealed and reacted at 90°C for 2h. After the reaction, the polymerization system was precipitated in acetone, filtered and washed three times with acetone to remove the catalyst, and dried in vacuum at 70°C. The obtained purple solid was the hyperbranched polycation electrolyte, poly-N-methacryloyloxyethyl -N-(α-Bromoisobutyryloxy)ethyl-N,N-dimethylammonium bromide, (PMEBDAB). The structure of PMEBDAB, including molecular weight and degree of branching, was used 1 H-NMR analysis, see accompanying drawing 1. The peaks with chemical shifts at 5.6 and 6.0 in the figure are protons of double bonds (CH 2 =); The peak of chemical shift at 3.6~3.9 is the methylene proton (O=C...
Embodiment 2
[0045] Embodiment 2: Utilize SCATRP of MEBDAB to prepare hyperbranched polycation electrolyte PMEBDAB
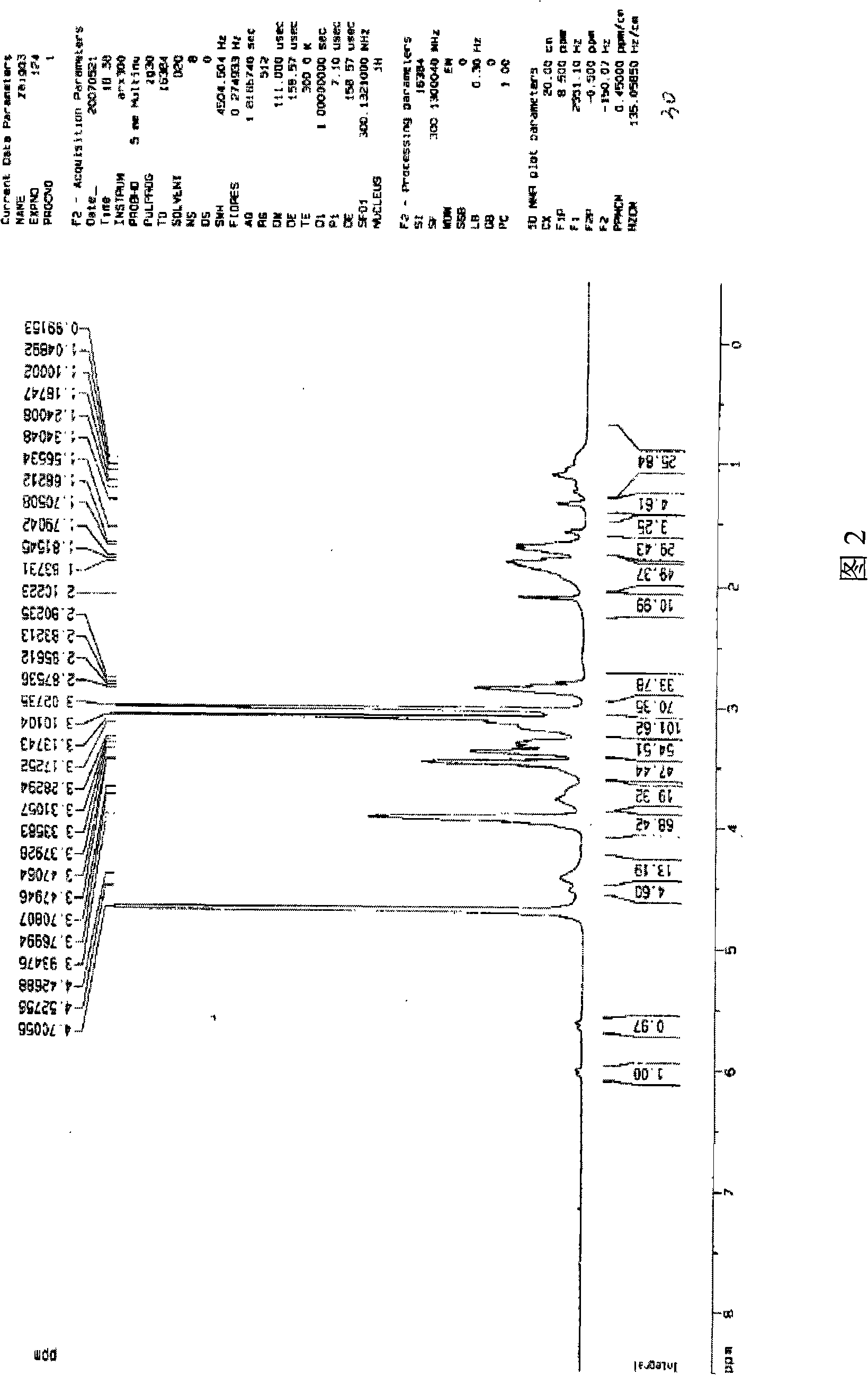
[0046] Add MEBDAB (4.31g, 10mmol), CuBr (14.4mg, 0.1mmol), PMDETA (34.6mg, 0.2mmol) and 8ml of water / ethanol (volume ratio 1 / 1) into a round bottom flask, repeatedly evacuate and fill with argon , and then sealed and reacted at 90°C for 24 hours. After the reaction, the polymerization system was precipitated in acetone, filtered and washed three times with acetone to remove the catalyst, and dried in vacuum at 70°C. The obtained purple solid was the hyperbranched polycation electrolyte, PMEBDAB. The structure of PMEBDAB, including molecular weight and degree of branching, was used 1 H-NMR analysis, see accompanying drawing 2. The peaks with chemical shifts at 5.6 and 6.0 in the figure are protons of double bonds (CH 2 =); The peak of chemical shift at 3.6~3.9 is the methylene proton (O=C-O-CH) that is connected with ester group 2 -); The peak with a chemical shift of 3.4...
Embodiment 3
[0047] Embodiment three: Utilize the SCATRP of MECDAC to prepare hyperbranched polycation electrolyte PMECDAC
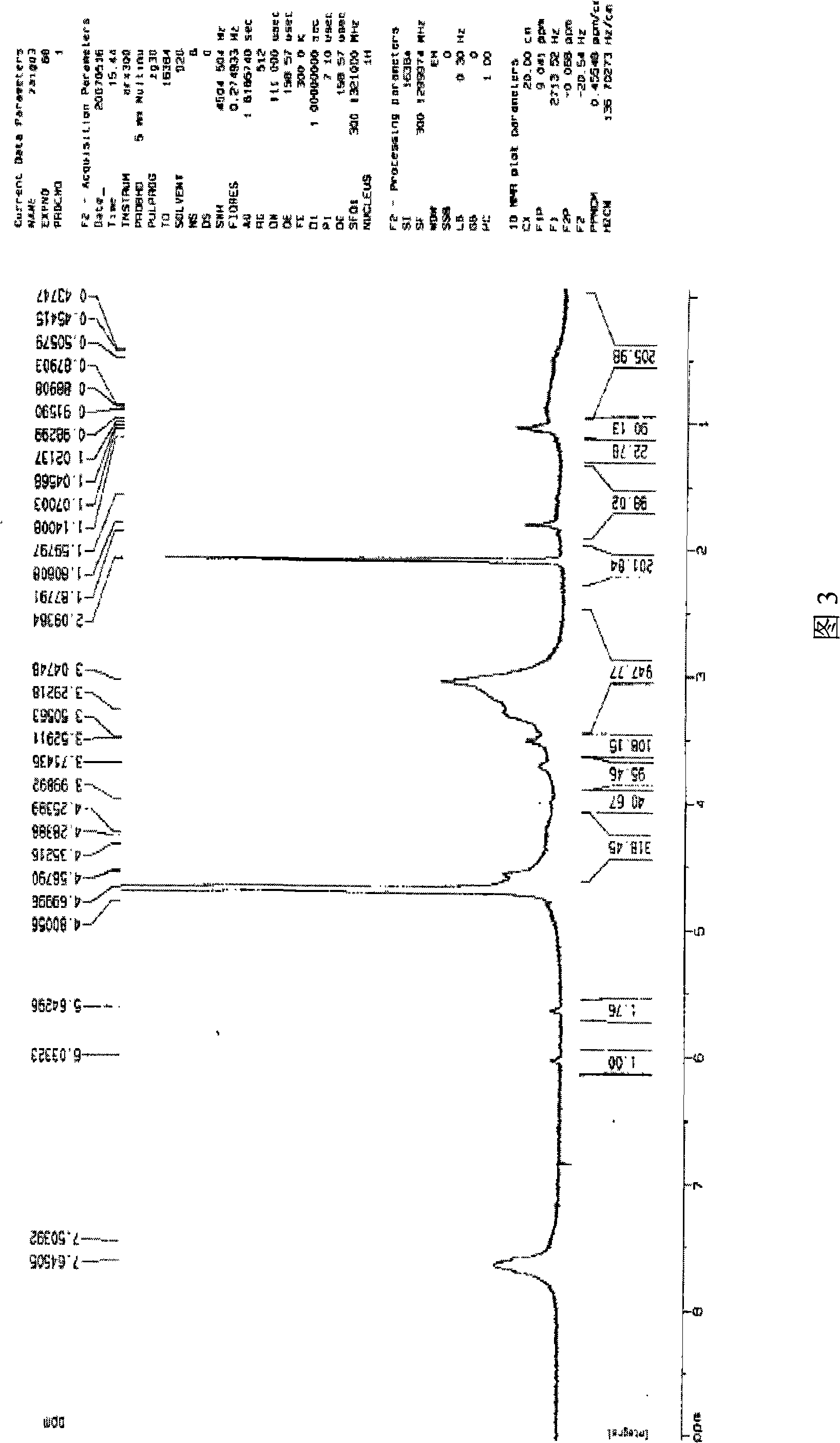
[0048] Add MECDAC (1.66g, 5mmol), CuCl (4.95mg, 0.05mmol), bPy (15.6mg, 0.1mmol) into 6ml of water / THF (volume ratio 1 / 1), repeatedly evacuate and inflate with argon, and then seal in React at 90°C for 18 hours. After the reaction, the polymerization system was precipitated in acetone, filtered and washed three times with acetone to remove the catalyst, and dried in vacuum at 70°C. The obtained white solid was the hyperbranched polycation electrolyte, PMECDAC. The product structure of PMECDAC, including molecular weight and degree of branching, was used 1 H-NMR analysis, see accompanying drawing 3. The peak with a chemical shift of 7.6 in the figure is the proton of the benzene ring (-C 6 h 4 -); The peaks with chemical shifts at 5.6 and 6.0 are double bond protons (CH 2 =); The peak with chemical shift at 4.6 is the methylene proton hydrogen (-CH 2 -C 6 h 4 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com