Azo-dyes congo layered hydrotalcite and preparation thereof
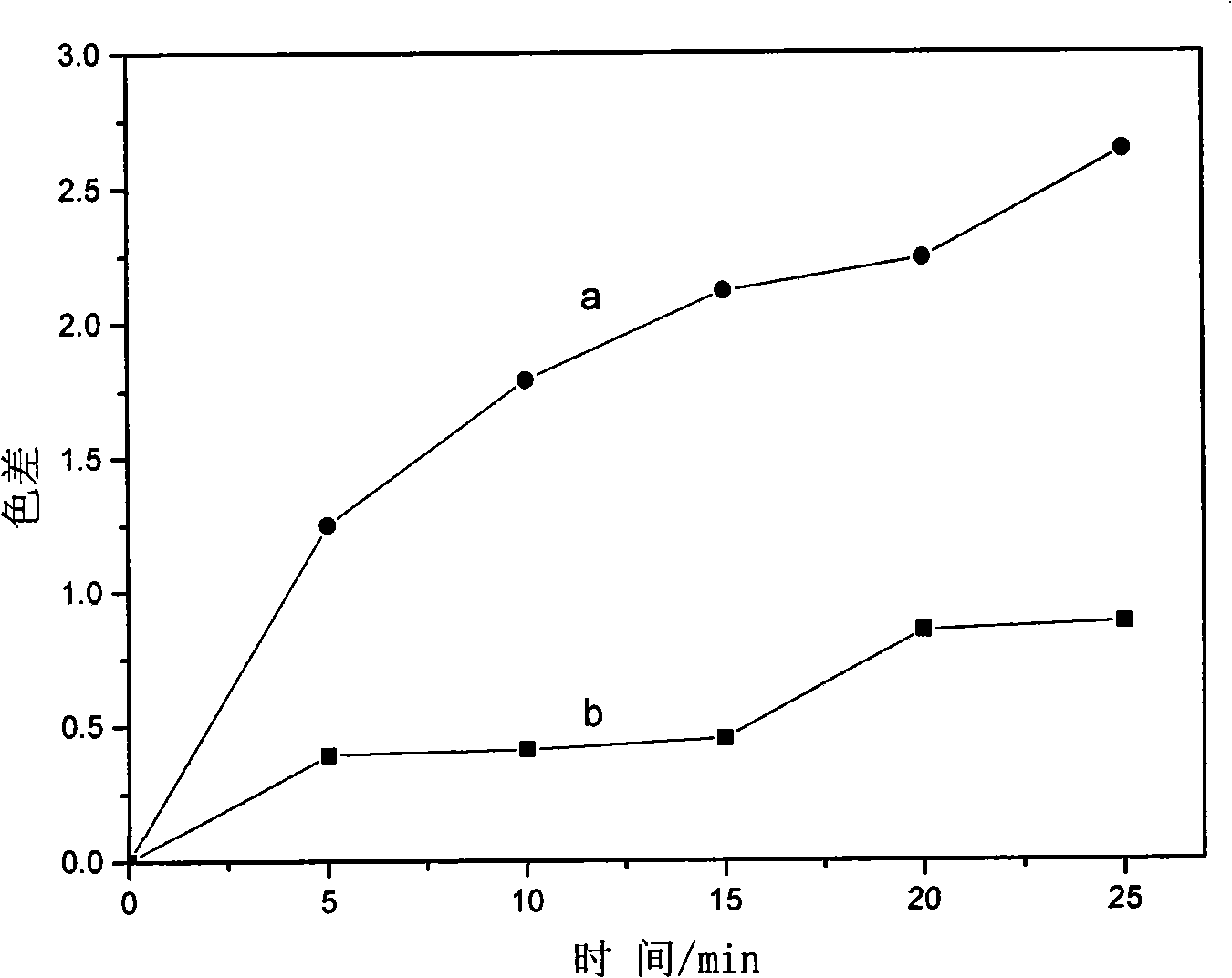
A technology of azo dyes and hydrotalcites, which is applied in the direction of azo dyes, azo dye complex metal compounds, organic dyes, etc., can solve the problems of easy decolorization and fading, poor light and heat stability, etc., to improve light and heat stability, The effect of improving heat resistance stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
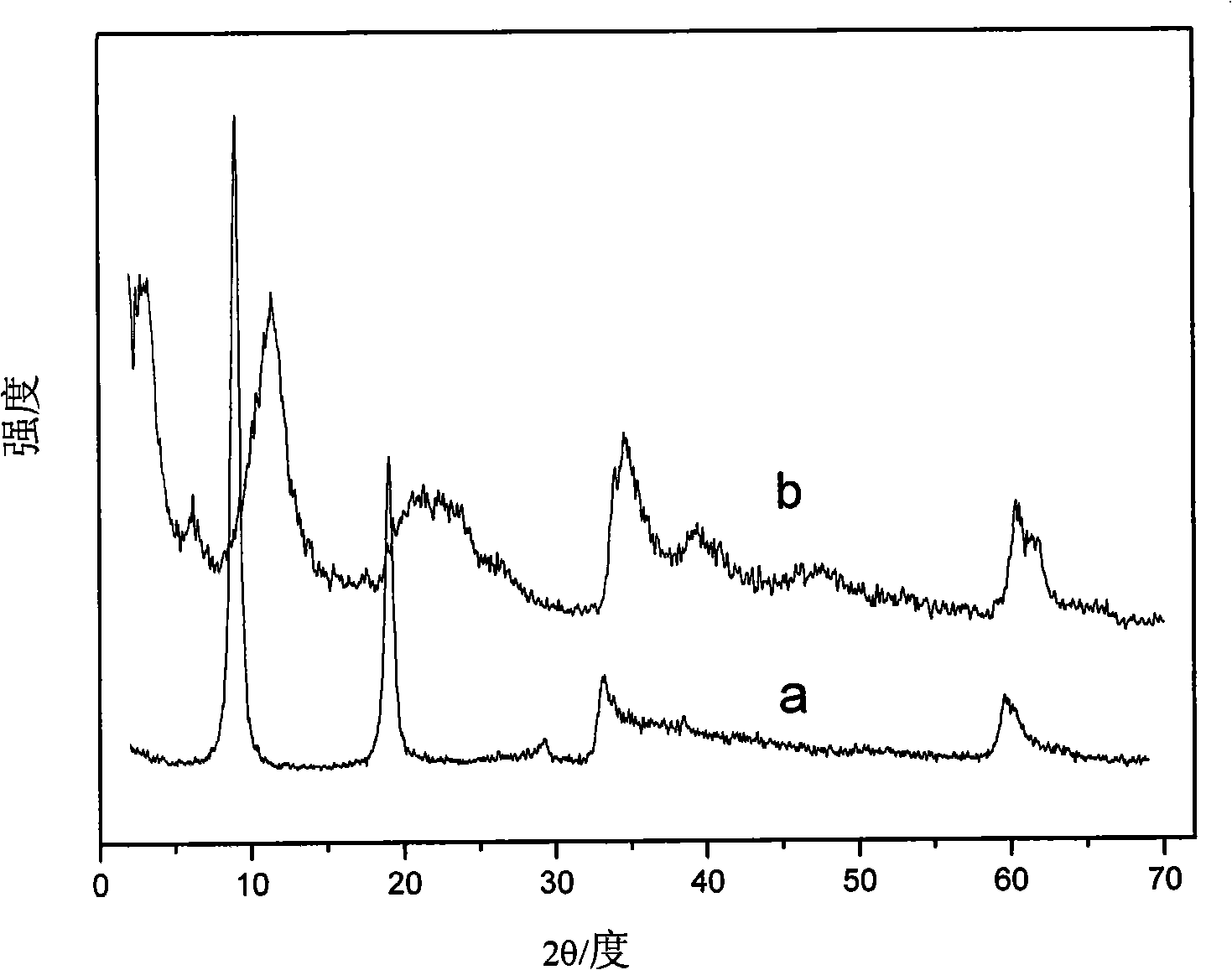
[0019] Step A: take by weighing 71.39g Zn(NO 3 ) 2 ·6H 2 O and 45.02g Al(NO 3 ) 3 9H 2 O dissolved in 200ml to remove CO 2 Mixed salt solution was prepared in deionized water, and another 27.36g NaOH was dissolved in 200ml deCO 2 Alkaline solution was prepared in deionized water. The prepared solution was rapidly mixed in a fully back-mixed rotating liquid film nucleation reactor, and after vigorous circulation and stirring for 2 minutes, the resulting slurry was placed in a reaction kettle for crystallization at 100° C. for 14 hours. deCO 2 The crystallized product was fully washed with deionized water to pH3 -LDHs precursor, whose Zn 2+ / Al 3+ =2.
[0020] Step B: According to the molar ratio of the azo dye Congo red and the hydrotalcite precursor being 2, first weigh 3.48g of Congo red and dissolve it in 40ml to remove CO 2 in deionized water; then weigh 4.06g of the filter cake prepared in step A and dissolve it in 60ml of deCO 2 In deionized water, oscillate i...
Embodiment 2
[0024] Step A: take by weighing 46.15g Mg(NO 3 ) 2 ·6H 2 O and 33.76g Al(NO 3 ) 3 9H 2 O dissolved in 150ml deCO 2 Prepare a mixed salt solution in deionized water, and dissolve another 21.60g NaOH in 150ml deCO 2 Alkaline solution was prepared in deionized water. The prepared solution was rapidly mixed in a fully back-mixed rotary liquid film nucleation reactor, and after vigorous circulation and stirring for 2 minutes, the obtained slurry was placed in a reaction kettle for crystallization at 100° C. for 4 hours. deCO 2 The crystallized product was fully washed with deionized water to pH3 -LDHs precursor, its Mg 2+ / Al 3+ =2.
[0025] Step B: According to the molar ratio of the azo dye Congo red and the hydrotalcite precursor being 2, first weigh 3.48g of Congo red and dissolve it in 40ml to remove CO 2 in deionized water; then weigh 5.12g of the filter cake prepared in step A and dissolve it in 60ml of deCO 2 In deionized water, oscillate in an ultrasonic genera...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com