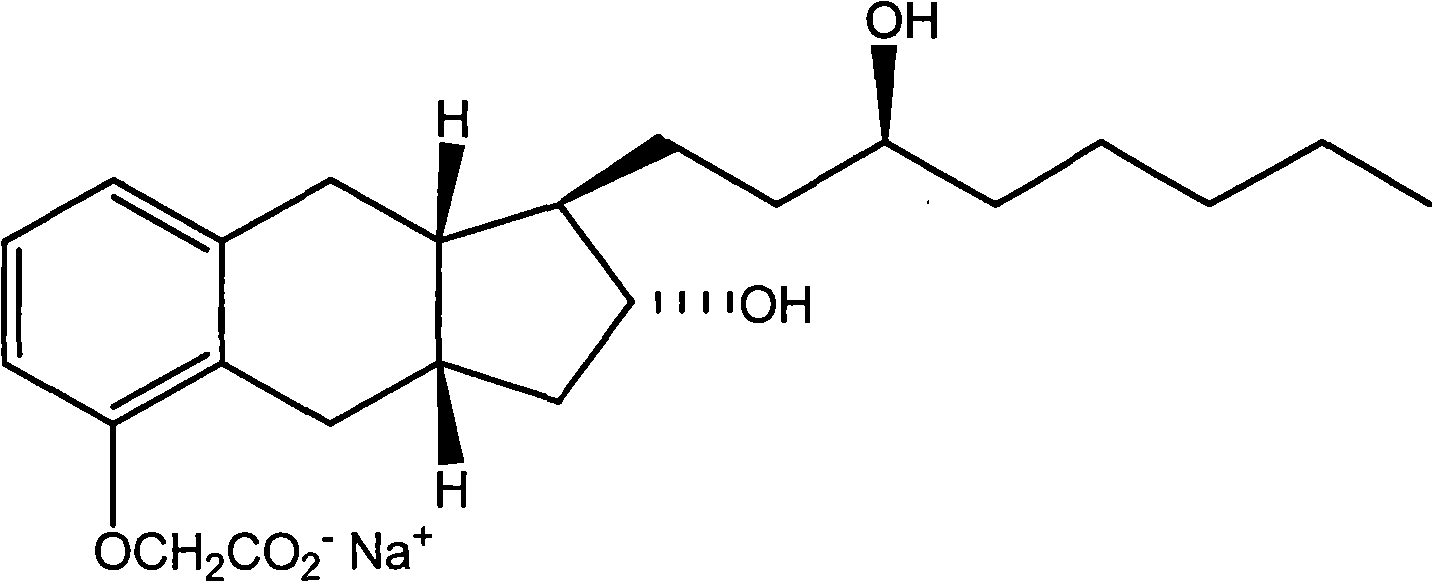
Use of treprostinil to treat neuropathic diabetic foot ulcers
A neuropathic, diabetic technology, applied in the treatment of foot ulcers in patients with diabetic neuropathy, halgh DG, can solve the problem of unsuccessful treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Treprostinil sodium (Remodulin ) Is safe and promotes diabetes and peripheral arterial disease The patient's ischemic wound healing and resting pain relief.
[0051] introduction: The purpose of this open-label study is to evaluate the safety of a stable prostaglandin analog, treprostinil sodium, in 10 patients with PAD, diabetes, and inoperable ischemic foot ulcers. And effectiveness.
[0052] method: Treprostinil sodium was given by continuous subcutaneous infusion, starting at 2.5ng / Kg / min and dripping to the highest tolerated dose, for 12 weeks. Monitor patients' adverse reactions, wound healing, skin perfusion pressure, and ischemic resting pain.
[0053] result: Seven patients have completed the experiment so far. No serious adverse reactions caused by treprostinil occurred during the experiment. The only reported drug-related side effect was mild to moderate pain at the infusion site. Two patients were completely cured. Two patients were partially cured. ...
Embodiment 2
[0056] Use Treprostinil sodium (Remodulin ) Enhance blood around trauma in ischemic limbs Oxygen quantification and laser Doppler signal
[0057] introduction: In diabetic patients with recalcitrant lower extremity trauma, a case-control trial was conducted by continuous subcutaneous infusion to check treprostinil sodium (Remodulin ). The known vasodilatation and platelet aggregation inhibitor properties of treprostinil sodium ensure that it can be used as an adjuvant drug in wound healing of ischemic limbs. The primary endpoint is wound healing and limb salvation. Among the secondary endpoints are resting ring trauma percutaneous oximetry (TcPO2) and laser Doppler analysis (LD), as well as the response of TcPO2 and LD to normal pressure oxygen or hyperbaric oxygen stimulation. If treprostinil sodium reduces the inflow resistance to the distal side of the capillary sphincter connecting the arterioles, between the trauma center with low oxygen content and the inflow blood...
Embodiment 3
[0065] Give Treprostinil to people with diabetic neuropathy and foot ulcers
[0066] Treprostinil was administered to patients with diabetic neuropathy who had at least one ulcer (ie, small ulcer or small area of tissue gangrene) on the foot in escalating doses over 12 weeks. The therapeutic drug is delivered through a small pump connected to a catheter placed under the skin. In this way, escalating doses of Treprostinil are continuously subcutaneously infused into the patient for a long time.
[0067] Specifically, Treprostinil sodium (REMODULIN) was administered subcutaneously using a standard micro-infusion, positive pressure infusion pump designed for subcutaneous drug delivery (Mini-Med) ) 1.0mg / mL formulation. The patient received an initial dose of 2.5 ng / kg / min of study drug. If the patient does not tolerate the 2.5ng / kg / min dose (such as persistent headache, nausea, vomiting, restlessness, anxiety, or severe pain at the infusion site that cannot be adequately treated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com