Expression process of heat shock protein Hsp16.3 of mycobacterium tuberculosis
A Mycobacterium tuberculosis, expression method technology, applied in the direction of microorganism-based methods, biochemical equipment and methods, bacteria, etc., can solve problems such as Hsp16.3 protein difficulties, and achieve the effect of broad-spectrum immune reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
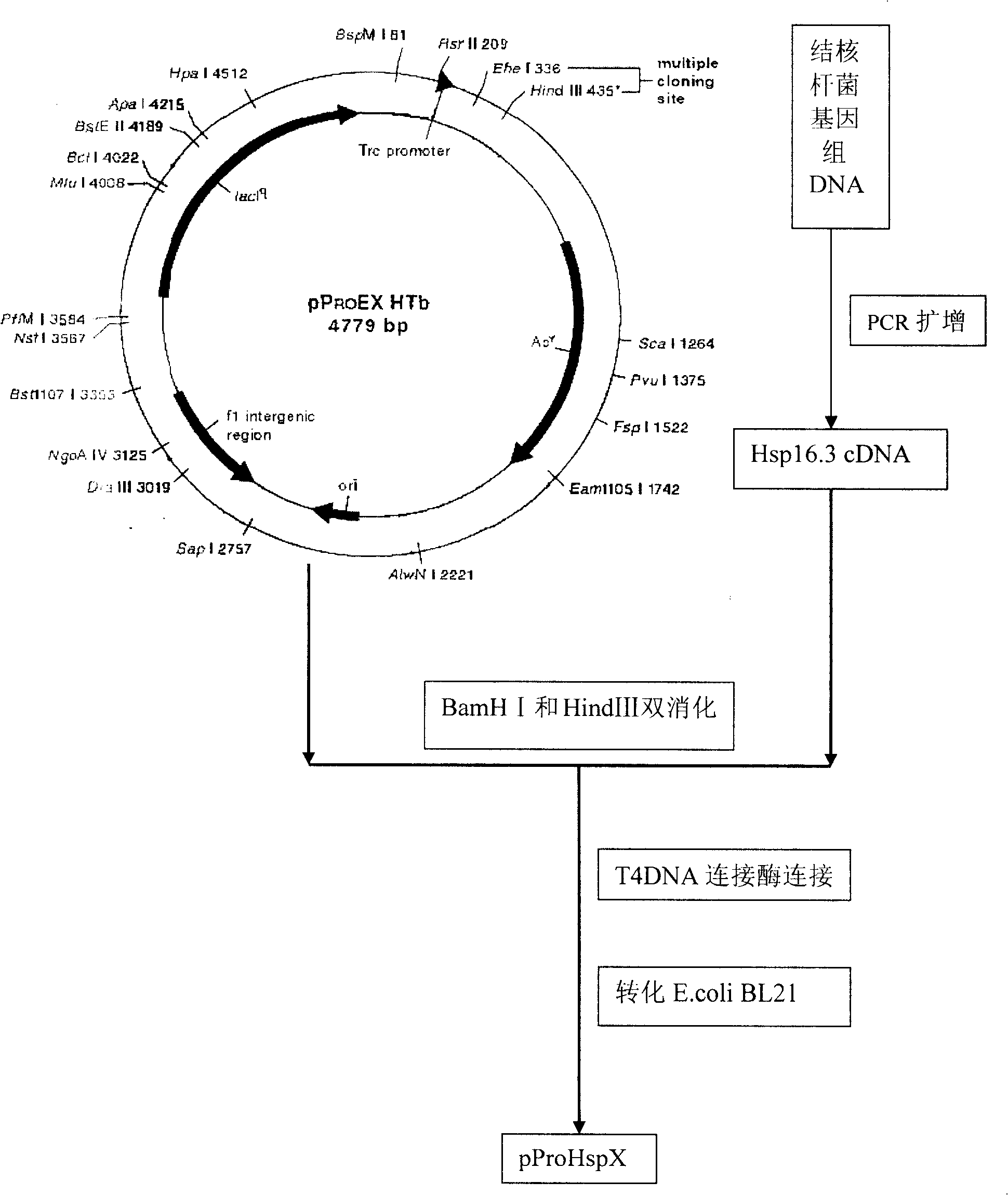
[0041] Embodiment 1, Construction and Identification of Recombinant Expression Plasmids
[0042] The pProEXHTb vector was digested with BamHI and HindIII, and recovered with a gel recovery kit after 1% agarose electrophoresis; the Hsp16.3 coding gene was amplified from the chromosomal DNA of Mycobacterium tuberculosis H37Rv by PCR, and the PCR product was digested with BamHI and HindIII. After 1% agarose electrophoresis, it was recovered with a gel recovery kit; the two digested products were connected with T4DNA ligase, transformed into Escherichia coli DH5α, and the recombinant plasmid pProHspX was extracted. Further enzyme digestion identification and sequence analysis proved that the constructed recombinant expression plasmid was completely correct. The recombinant plasmid pProHspX was further transformed into Escherichia coli BL21 to obtain a genetic engineering expression strain of the recombinant protein Hsp16.3.
Embodiment 2
[0043] Embodiment 2, induced expression of recombinant engineering strain
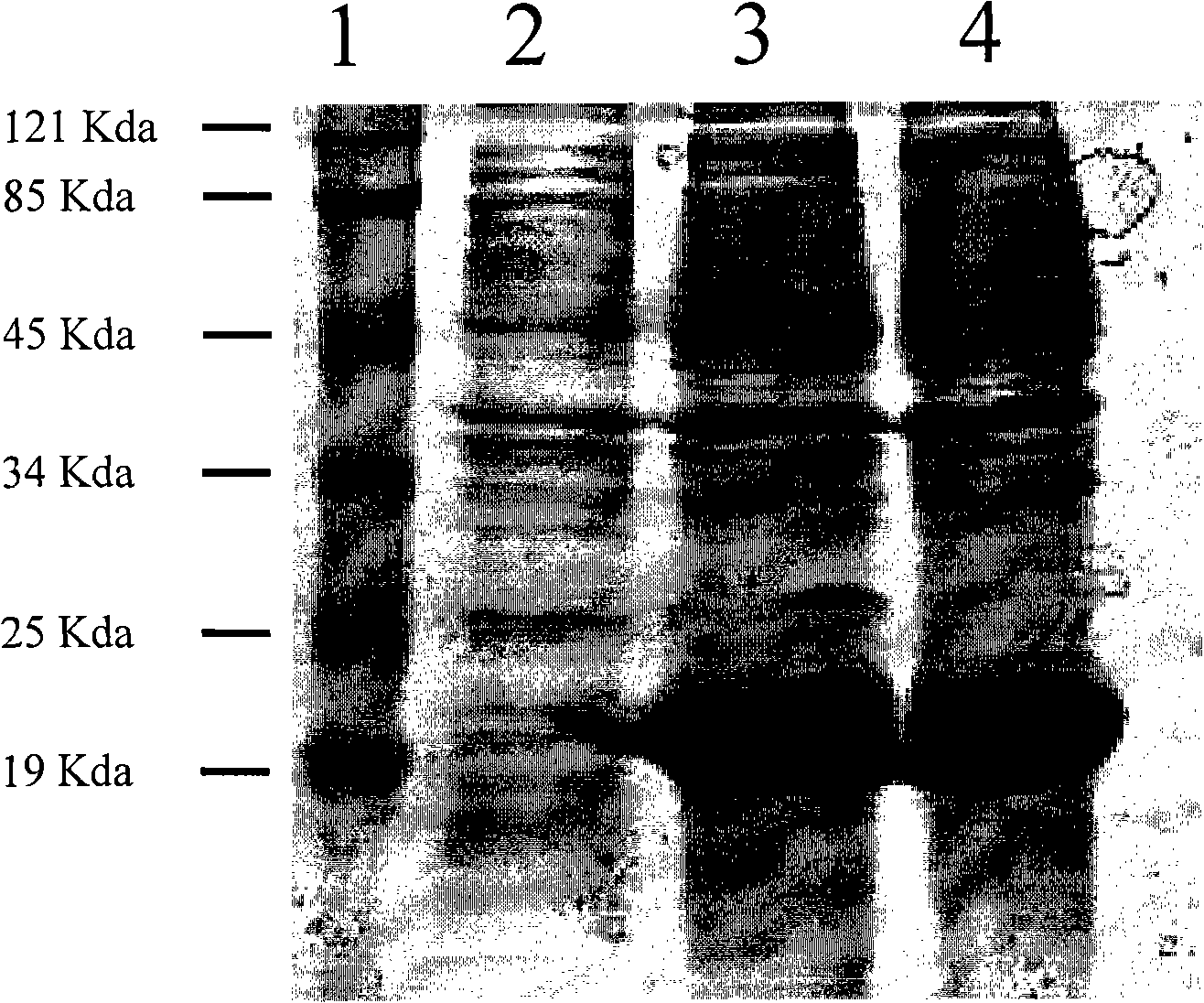
[0044]The Escherichia coli BL21 strain transformed with the recombinant plasmid pProHspX was shaken overnight in LB medium at 37°C (containing ampicillin, final concentration 100 μg / ml); the next day, the bacteria were collected by centrifugation and placed in 1L LB medium at a ratio of 1:100 Continue shaking and culturing until OD600 reaches 0.6; add IPTG with a final concentration of 1 mM, and continue culturing for 4-6 hours. Take 1.5ml of the bacteria before and after induction, centrifuge, remove the supernatant, add 2×SDS sample buffer to the bacterial pellet, boil at 100°C for 5min, and detect with 12% SDS-PAGE. The results confirmed that the high expression strain of Hsp16.3 protein was obtained, and the expression level was as high as 200mg / L fermentation broth.
Embodiment 3
[0045] The western blot identification of embodiment 3 expression products
[0046] The SDS-PAGE gel after electrophoresis was stripped, and electrotransferred to the NC membrane under the condition of 200mA constant current for 2h; the transferred NC membrane was placed in 1% BSA-PBS blocking solution, shaken at 37°C for 1h, and washed with 0.05% Tween20-PBS (PBST) solution was shaken at room temperature and washed 3 times; 3ml of tuberculosis patient serum was used as the primary antibody, incubated at room temperature for 1h, and washed 3 times with PBST solution at room temperature with shaking; horseradish peroxidase-labeled goat anti-human IgG antibody ( Diluted with blocking solution (1:10000 fold) as the secondary antibody, incubated for 1 h, washed with PBST solution for 3 times with shaking at room temperature; developed color by chemiluminescence in a dark room. The results confirmed the specific expression of a protein with a molecular weight of about 19kDa.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com