Alpha1-receptor antagonist slow-release pill preparation and its preparation method
A technology of receptor antagonists and sustained-release pellets, which can be applied to medical preparations containing active ingredients, pill delivery, anti-tumor drugs, etc., and can solve problems such as unrealized and unavailable tamsulosin sustained-release curves
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: Preparation of slow-release pellet preparation
[0046] (1) Preparation of pellets containing active ingredients
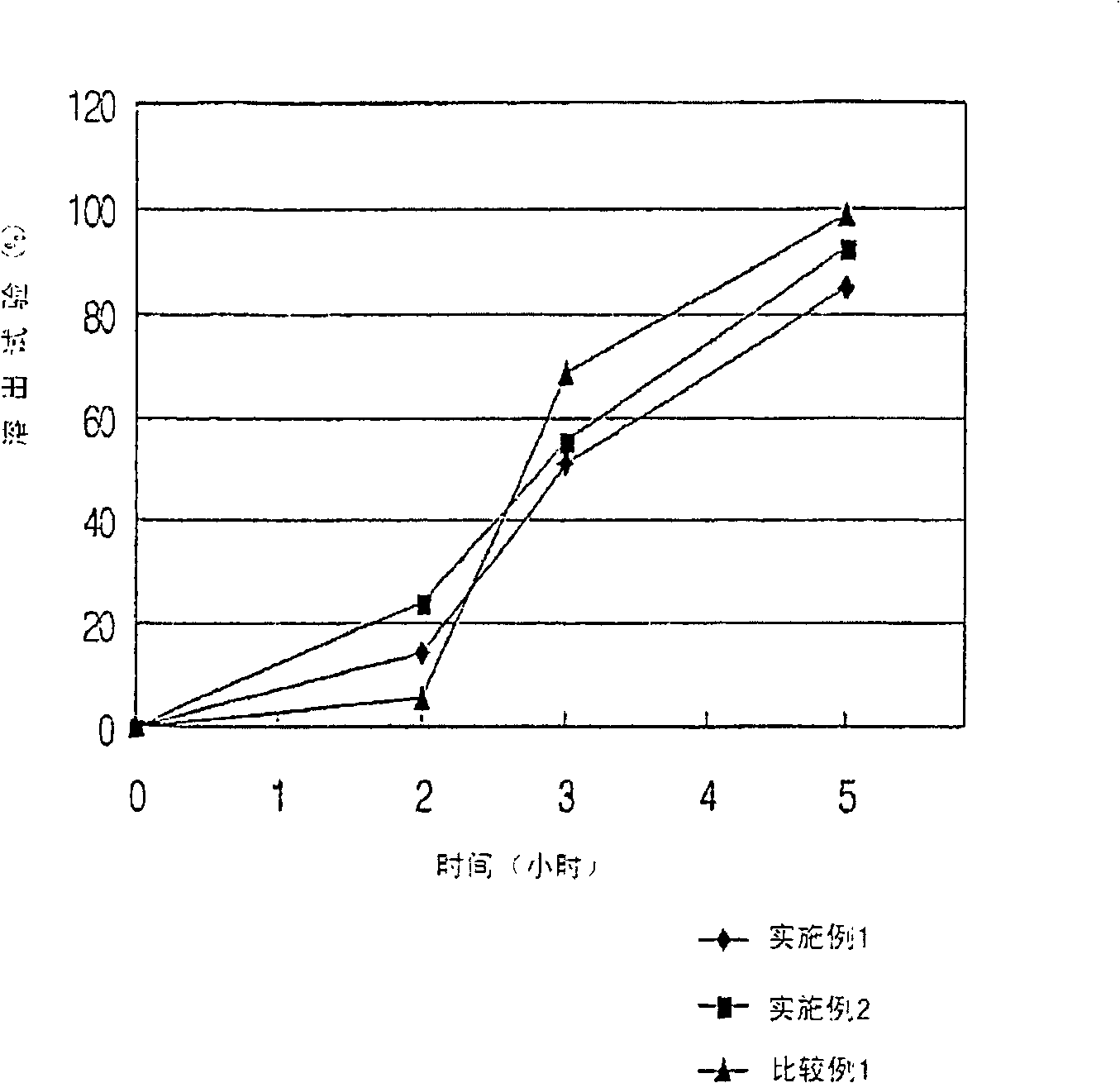
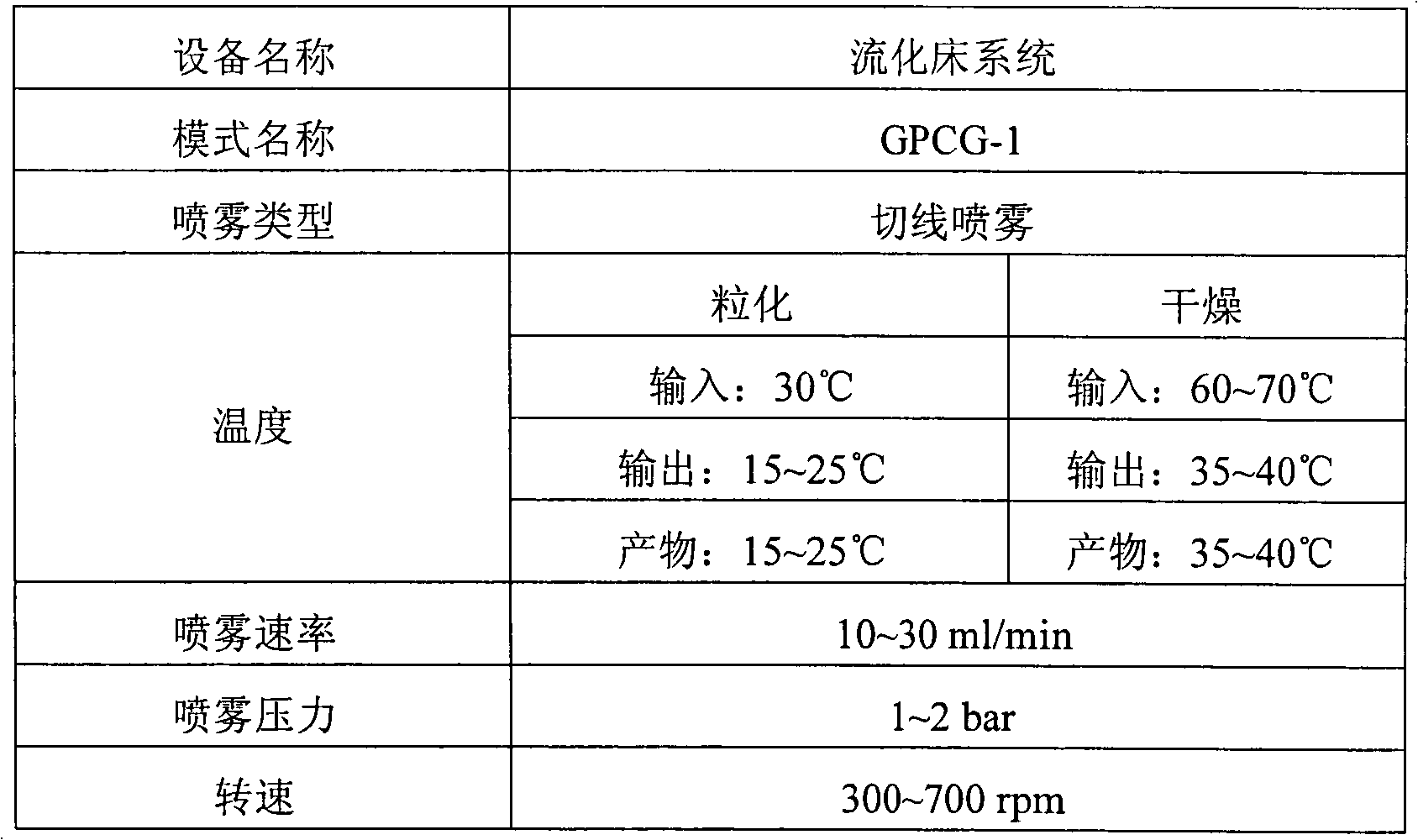
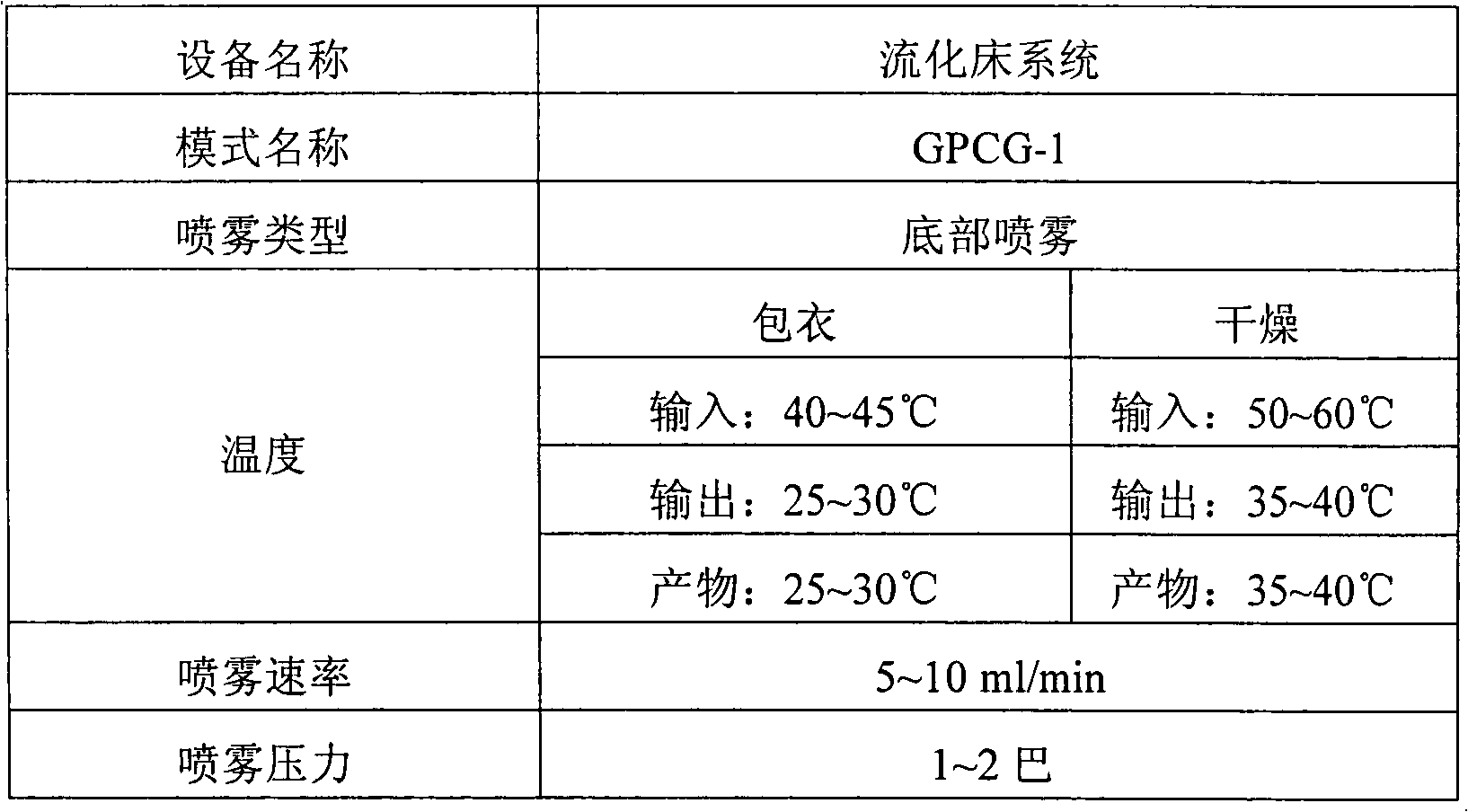
[0047] 1.0 g of tamsulosin hydrochloride (Ragactives, Spain), 747 g of microcrystalline cellulose and 16 g of talc were thoroughly mixed in a centrifugal fluidized bed granulator (GPCG-1, Glatt, Germany) for about 1 minute. The binder solution (120g Eudragit in 580g water TM L30D-55) is sprayed onto the mixture in the granulator to form pellets. The pellet preparation conditions are listed in Table 1. The pellets thus obtained were spherical particles with a diameter of 0.5 to 1.4 mm, as shown in Table 2.
[0048]
[0049]
[0050]
[0051] Particle size (μm)
Weight (g)
Proportion(%)
>1400
0.45
0.45
710~1400
9.77
9.76
[0052] 500~710
88.39
88.34
355~500
1.45
1.45
<355
-
-
total
100.06 ...
Embodiment 2
[0059] Embodiment 2: Preparation of slow-release pellet preparation
[0060] (1) Preparation of pellets containing active ingredients
[0061] 24.25 g of doxazosin mesylate (Cipla, India), 400 g of microcrystalline cellulose, 295.75 g of calcium hydrogen phosphate, and 40 g of talc were fully granulated in a centrifugal fluidized bed granulator (GPCG-1, Glatt, Germany). Mix for about 1 minute. The binder solution (133.3g Eudragit in 600g water TM L30D-55) is sprayed onto the mixture in the granulator to form pellets. The pellet preparation conditions are listed in Table 1. The pellets thus obtained were spherical particles with a diameter ranging from 0.5 to 1.4 mm, as shown in Table 5.
[0062]
[0063] Particle size (μm)
Weight (g)
Proportion(%)
>1400
8.78
8.79
710~1400
79.22
79.28
500~710
11.41
11.42
355~500
0.52
0.52
<355
-
-
total
...
Embodiment 3
[0068] Embodiment 3: Preparation of slow-release pellet preparation
[0069] (1) Preparation of pellets containing active ingredients
[0070] 50 g of alfuzosin hydrochloride (Heumann PCS, Germany), 550 g of microcrystalline cellulose, 120 g of calcium hydrogen phosphate, and 40 g of talc were thoroughly mixed in a centrifugal fluidized bed granulator (GPCG-1, Glatt, Germany) for about 1 minute. The binder solution (133.3g Eudragit in 600g water TM L30D-55) is sprayed onto the mixture in the granulator to form pellets. The pellet preparation conditions are listed in Table 1. The pellets thus obtained were spherical particles with a diameter ranging from 0.7 to 1.4 mm, as shown in Table 7.
[0071]
[0072] Particle size (μm)
Weight (g)
Proportion(%)
>1400
4.48
4.48
710~1400
91.96
92.04
500~710
3.24
3.24
355~500
0.23
0.23
<355
-
-
total
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com