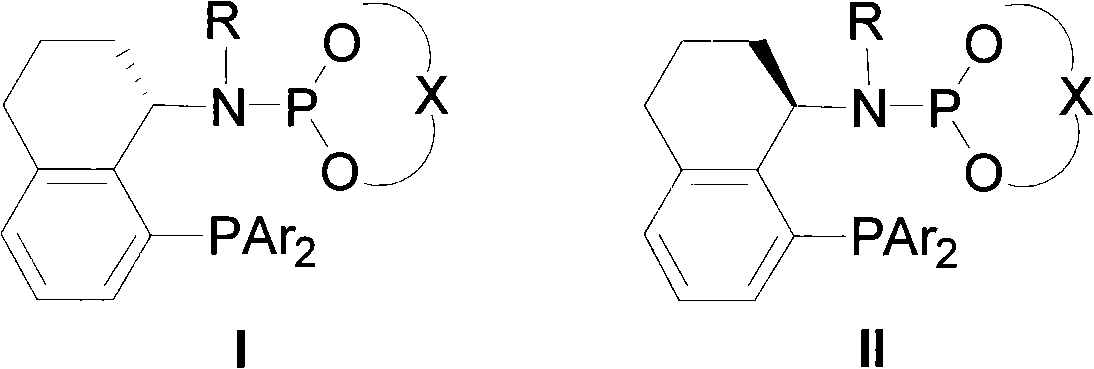
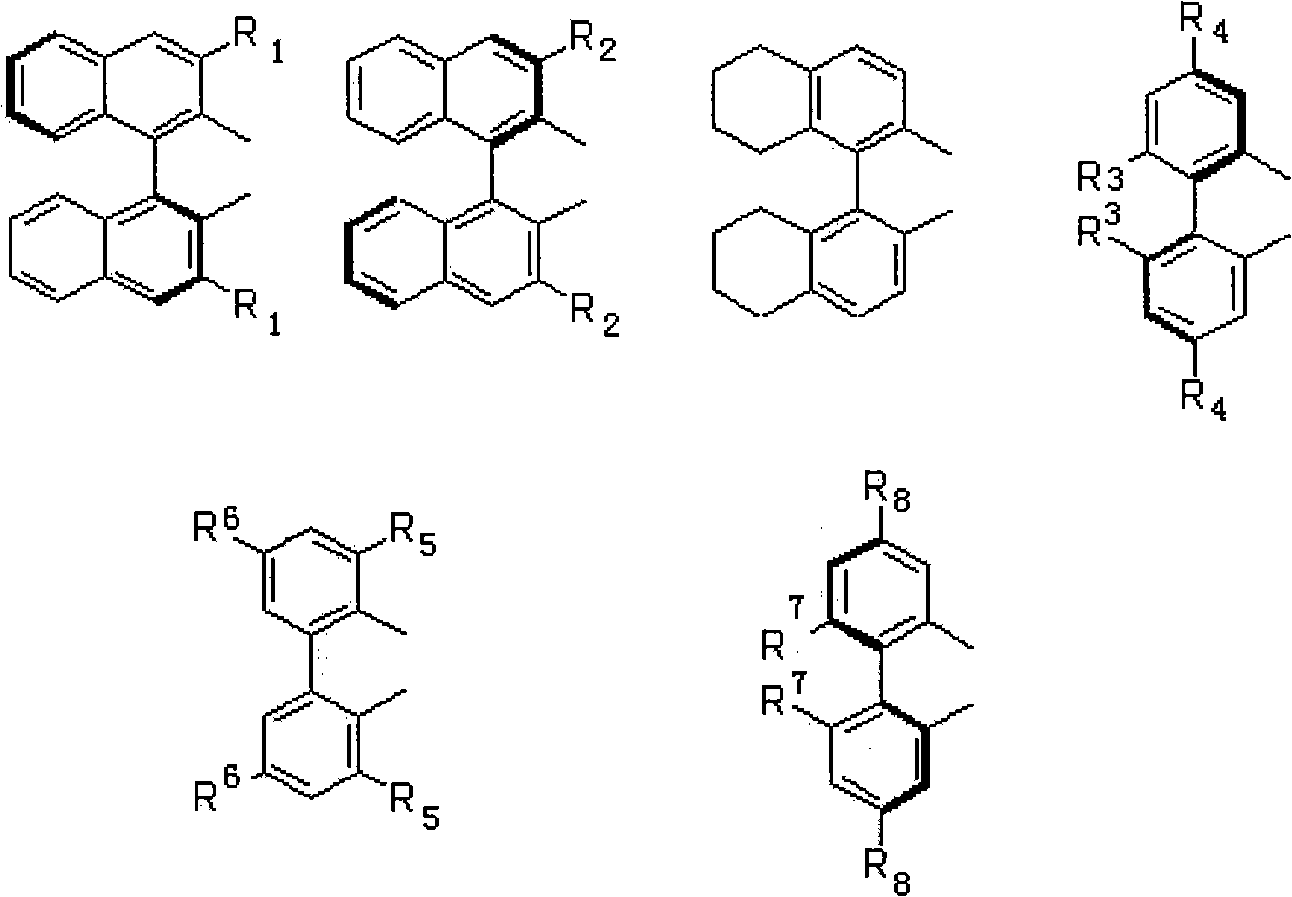
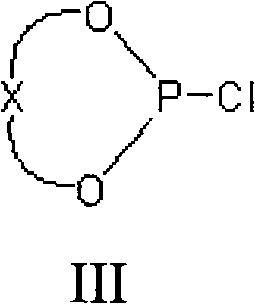Asymmetric catalytic reaction catalyst and preparation and use thereof
A catalytic reaction, asymmetric technology, applied in the direction of physical/chemical process catalysts, asymmetric synthesis, organic chemical methods, etc., can solve the problems of incomplete conversion of reaction substrates, low yield of economically feasible catalysts, etc., and achieve catalytic activity High, high catalytic activity, high optical selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1. the synthesis of chiral ligand intermediate A (phosphine-primary amine compound)
[0060] The structural formula of the chiral ligand intermediate A is as follows:
[0061]
[0062] Chiral ligand intermediate A is prepared according to the following method:
[0063] Add 1.47 g of (R)-1,2,3,4-tetrahydro-1-naphthylamine and 10 ml of ether into a 100 ml three-necked flask, and slowly add 5.5 ml of n-BuLi with a concentration of 2.0 mol / l at 0°C hexane solution. After the addition was complete, the stirring reaction was continued at 0° C. for 15 minutes, and then 1.09 g of trimethylchlorosilane was added. After reacting for 1 hour, 16.5 ml of n-BuLi hexane solution with a concentration of 2.0 mol / l was added slowly, and the reaction mixture was slowly raised to room temperature within 5 hours. After reacting for 1 hour, the reaction night was cooled to -20°C, and a solution formed of 2.2 g of diphenylphosphine chloride and 10 ml of ether was added slowly...
Embodiment 2
[0064] Example 2. Synthesis of chiral ligand B (phosphine-phosphoramidite ligand)
[0065] The structural formula of chiral ligand B is as follows:
[0066]
[0067] Chiral ligand B was prepared as follows:
[0068] 1) Add 10 grams of (R)-BINOL ((R)-2,2'-dihydroxy-1,1'-binaphthalene) and 75 grams of PCl to a 100ml three-neck flask 3 And a catalytic amount of 2-methylpyrrolidone, the reaction was refluxed until the solid disappeared (about 10 minutes). Remove most of PCl under reduced pressure under 30mmHg vacuum 3 , a small amount of residual PCl 3 Azeotropic removal under reduced pressure with 50ml of toluene. After the toluene was removed, the residue was recrystallized with 30 ml of n-hexane to obtain 11.9 g of white chlorophosphite.
[0069] 2) Add 3.51 grams of phosphorochloride prepared in step 1) and 30 ml of anhydrous toluene into a 100 ml three-necked flask, and slowly add 3.31 grams of A prepared in Example 1 and 3.03 grams of triethylamine to dissolve at 0°C...
Embodiment 3
[0070] Example 3. Synthesis of chiral ligand C (phosphine-phosphoramidite ligand)
[0071] The structural formula of the chiral ligand C is as follows:
[0072]
[0073] Chiral ligand C was prepared as follows:
[0074] 1) Add 10 grams of (S)-BINOL and 75 grams of PCl into a 100ml three-necked bottle 3 And a catalytic amount of 2-methylpyrrolidone, the reaction was refluxed until the solid disappeared (about 10 minutes). Remove most of PCl under reduced pressure 3 , a small amount of residual PCl 3 Azeotropic removal under reduced pressure with 50ml of toluene. After the toluene was removed, the residue was recrystallized with 30 ml of n-hexane to obtain 11.5 g of white chlorophosphite.
[0075] 2) Add 3.51 grams of phosphorochloride prepared in step 1) and 30 ml of anhydrous toluene into a 100 ml three-necked flask, and slowly add 3.31 grams of A prepared in Example 1 and 3.03 grams of triethylamine to dissolve at 0°C. A solution formed in 20ml of toluene. After the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com